|
Fukuyama Coupling
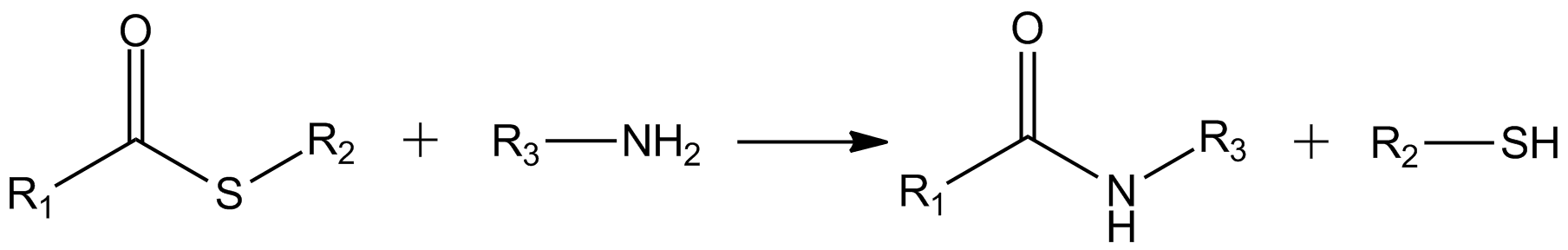
The Fukuyama coupling is a coupling reaction taking place between a thioester and an organozinc halide in the presence of a palladium catalyst. The reaction product is a ketone. This reaction was discovered by Tohru Fukuyama et al. in 1998. Advantages are high chemoselectivity, mild reaction conditions and the use of less-toxic reagents. : One advantage of this method is that the reaction stops at the ketone and does not proceed to a tertiary alcohol. In addition, the protocol is compatible with functional groups In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ... such as ketones, acetates, sulfides, aromatic bromides, chlorides and aldehydes. : The reaction (interrupted) has been used in the synthesis of biotin : This reaction was preceded by the conceptually related Fukuy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tohru Fukuyama
is a Japanese organic chemist and Professor of Chemistry at University of Tokyo in Japan. He discovered the Fukuyama coupling in 1998. Biography Fukuyama studied chemistry at Nagoya University with degrees Bachelor's (1971) and Master's (1973) degrees. As a graduate student, he then worked at Harvard University, where he received his doctorate in 1977 as an academic student of Yoshito Kishi. Until 1978, he continued his research as a postdoc in the Department of Chemistry of Harvard University and then moved to Rice University as an assistant professor, where in 1988 he obtained the rank of a chair holder. In 1995, he accepted a professorship in Pharmaceutical Sciences from the University of Tokyo, Japan. Since 2013, Fukuyama has been working as a professor at the Nagoya University - more precisely: Designated Professor of Pharmaceutical Sciences. The 2015 Nobel Prize in Physiology or Medicine winner Satoshi Ōmura is his old friend. Achievements *Fukuyama reduction *Fukuyama in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coupling Reaction
A coupling reaction in organic chemistry is a general term for a variety of reactions where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = main group center) reacts with an organic halide of the type R'-X with formation of a new carbon-carbon bond in the product R-R'. The most common type of coupling reaction is the cross coupling reaction. Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki were awarded the 2010 Nobel Prize in Chemistry for developing palladium-catalyzed cross coupling reactions. Broadly speaking, two types of coupling reactions are recognized: *Heterocouplings combine two different partners, such as in the Heck reaction of an alkene (RC=CH) and an alkyl halide (R'-X) to give a substituted alkene, or the Corey–House synthesis of an alkane by the reaction of a lithium diorganylcuprate (R2CuLi) with an organyl (pseudo)halide (R'X ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioester
In organic chemistry, thioesters are organosulfur compounds with the functional group . They are analogous to carboxylate esters () with the sulfur in the thioester playing the role of the linking oxygen in the carboxylate ester, as implied by the ''thio-'' prefix. They are the product of esterification between a carboxylic acid () and a thiol (). In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA.Matthys J. Janssen "Carboxylic Acids and Esters" in PATAI's Chemistry of Functional Groups: Carboxylic Acids and Esters, Saul Patai, Ed. John Wiley, 1969, New York: pp. 705–764. Synthesis The most typical route to thioester involves the reaction of an acid chloride with an alkali metal salt of a thiol: :RSNa + R'COCl -> R'COSR + NaCl Another common route entails the displacement of halides by the alkali metal salt of a thiocarboxylic acid. For example, thioacetate esters are commonly prepared by alkylation of potassium thioacetate: :CH3COSK + ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organozinc Halide
Organozinc compounds in organic chemistry contain carbon (C) to zinc (Zn) chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.The Chemistry of Organozinc Compounds' (Patai Series, (Eds. Z. Rappoport and I. Marek), John Wiley & Sons: Chichester, UK, 2006, .''Organozinc reagents – A Practical Approach'', (Eds. P. Knochel and P. Jones), Oxford Medical Publications, Oxford, 1999, . Organozinc compounds were among the first organometallic compounds made. They are less reactive than many other analogous organometallic reagents, such as Grignard and organolithium reagents. In 1848 Edward Frankland prepared the first organozinc compound, diethylzinc, by heating ethyl iodide in the presence of zinc metal.E. Frankland, Liebigs Ann. Chem.,1849, 71, 171 This reaction produced a volatile colorless liquid that spontaneous combusted upon contact with air. Due to their pyrophoric nature, organozinc compounds a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas. Palladium, platinum, rhodium, ruthenium, iridium and osmium form a group of elements referred to as the platinum group metals (PGMs). They have similar chemical properties, but palladium has the lowest melting point and is the least dense of them. More than half the supply of palladium and its congener platinum is used in catalytic converters, which convert as much as 90% of the harmful gases in automobile exhaust (hydrocarbons, carbon monoxide, and nitrogen dioxide) into nontoxic substances (nitrogen, carbon dioxide and water vapor). Palladium is also used in electronics, dentistry, medicine, hydrogen purification, chemical applications, groundwate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedron Letters
''Tetrahedron Letters'' is a weekly international journal for rapid publication of full original research papers in the field of organic chemistry. According to the ''Journal Citation Reports'', the journal has a 2020 impact factor of 2.415. Indexing ''Tetrahedron Letters'' is indexed in: References See also *''Tetrahedron In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the o ...'' *'' Tetrahedron: Asymmetry'' Chemistry journals Weekly journals Publications established in 1959 Elsevier academic journals {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Groups
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. Functi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biotin
Biotin (or vitamin B7) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. The name ''biotin'', borrowed from the German , derives from the Ancient Greek word (; 'life') and the suffix "-in" (a suffix used in chemistry usually to indicate 'forming'). Chemical description Biotin is classified as a heterocyclic compound, with a sulfur-containing ring fused ureido and tetrahydrothiophene group. A C5-carboxylic acid side chain is appended to one of the rings. The ureido ring, containing the −N−CO−N− group, serves as the carbon dioxide carrier in carboxylation reactions. Biotin is a coenzyme for five carboxylase enzymes, which are involved in the catabolism of amino acids and fatty acids, synthesis of fatty acids, and gluconeogenesis. Biotinylation of histone proteins in nuclear chromatin plays a role in chromatin stability and g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biotin2000 Synthesis
Biotin (or vitamin B7) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. The name ''biotin'', borrowed from the German , derives from the Ancient Greek word (; 'life') and the suffix "-in" (a suffix used in chemistry usually to indicate 'forming'). Chemical description Biotin is classified as a heterocyclic compound, with a sulfur-containing ring fused ureido and tetrahydrothiophene group. A C5-carboxylic acid side chain is appended to one of the rings. The ureido ring, containing the −N−CO−N− group, serves as the carbon dioxide carrier in carboxylation reactions. Biotin is a coenzyme for five carboxylase enzymes, which are involved in the catabolism of amino acids and fatty acids, synthesis of fatty acids, and gluconeogenesis. Biotinylation of histone proteins in nuclear chromatin plays a role in chromatin stability and g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


2.jpg)