ATP Generation on:
[Wikipedia]
[Google]
[Amazon]
 Oxidative phosphorylation (UK , US ) or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize
Oxidative phosphorylation (UK , US ) or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize
 The electron transport chain carries both protons and electrons, passing electrons from donors to acceptors, and transporting protons across a membrane. These processes use both soluble and protein-bound transfer molecules. In mitochondria, electrons are transferred within the intermembrane space by the water- soluble electron transfer protein
The electron transport chain carries both protons and electrons, passing electrons from donors to acceptors, and transporting protons across a membrane. These processes use both soluble and protein-bound transfer molecules. In mitochondria, electrons are transferred within the intermembrane space by the water- soluble electron transfer protein
 NADH-coenzyme Q oxidoreductase, also known as ''NADH dehydrogenase'' or ''complex I'', is the first protein in the electron transport chain. Complex I is a giant enzyme with the mammalian complex I having 46 subunits and a molecular mass of about 1,000
NADH-coenzyme Q oxidoreductase, also known as ''NADH dehydrogenase'' or ''complex I'', is the first protein in the electron transport chain. Complex I is a giant enzyme with the mammalian complex I having 46 subunits and a molecular mass of about 1,000
 Succinate-Q oxidoreductase, also known as ''complex II'' or ''succinate dehydrogenase'', is a second entry point to the electron transport chain. It is unusual because it is the only enzyme that is part of both the citric acid cycle and the electron transport chain. Complex II consists of four protein subunits and contains a bound
Succinate-Q oxidoreductase, also known as ''complex II'' or ''succinate dehydrogenase'', is a second entry point to the electron transport chain. It is unusual because it is the only enzyme that is part of both the citric acid cycle and the electron transport chain. Complex II consists of four protein subunits and contains a bound
 Q-cytochrome c oxidoreductase is also known as ''cytochrome c reductase'', ''cytochrome bc1 complex'', or simply ''complex III''. In mammals, this enzyme is a
Q-cytochrome c oxidoreductase is also known as ''cytochrome c reductase'', ''cytochrome bc1 complex'', or simply ''complex III''. In mammals, this enzyme is a
 Cytochrome c oxidase, also known as ''complex IV'', is the final protein complex in the electron transport chain. The mammalian enzyme has an extremely complicated structure and contains 13 subunits, two heme groups, as well as multiple metal ion cofactors – in all, three atoms of copper, one of magnesium and one of zinc.
This enzyme mediates the final reaction in the electron transport chain and transfers electrons to oxygen and hydrogen (protons), while pumping protons across the membrane. The final
Cytochrome c oxidase, also known as ''complex IV'', is the final protein complex in the electron transport chain. The mammalian enzyme has an extremely complicated structure and contains 13 subunits, two heme groups, as well as multiple metal ion cofactors – in all, three atoms of copper, one of magnesium and one of zinc.
This enzyme mediates the final reaction in the electron transport chain and transfers electrons to oxygen and hydrogen (protons), while pumping protons across the membrane. The final
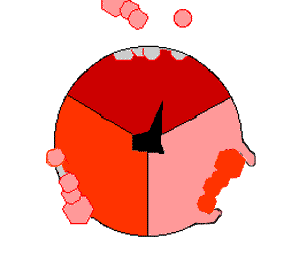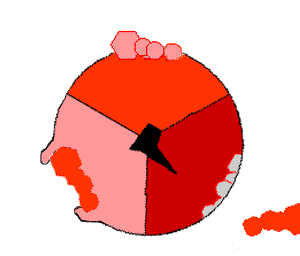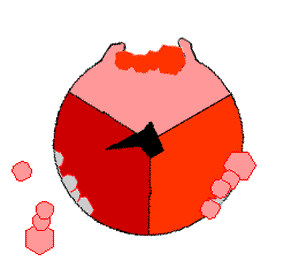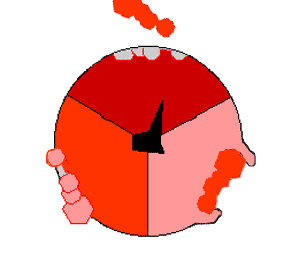 This ATP synthesis reaction is called the ''binding change mechanism'' and involves the active site of a β subunit cycling between three states. In the "open" state, ADP and phosphate enter the active site (shown in brown in the diagram). The protein then closes up around the molecules and binds them loosely – the "loose" state (shown in red). The enzyme then changes shape again and forces these molecules together, with the active site in the resulting "tight" state (shown in pink) binding the newly produced ATP molecule with very high
This ATP synthesis reaction is called the ''binding change mechanism'' and involves the active site of a β subunit cycling between three states. In the "open" state, ADP and phosphate enter the active site (shown in brown in the diagram). The protein then closes up around the molecules and binds them loosely – the "loose" state (shown in red). The enzyme then changes shape again and forces these molecules together, with the active site in the resulting "tight" state (shown in pink) binding the newly produced ATP molecule with very high
Animated diagrams illustrating oxidative phosphorylation
Wiley and Co ''Concepts in Biochemistry''
On-line biophysics lectures
Antony Crofts, University of Illinois at Urbana–Champaign
ATP Synthase
Graham Johnson
ATP synthase
*Interactive molecular models at Universidade Fernando Pessoa:
NADH dehydrogenase
{{DEFAULTSORT:Oxidative Phosphorylation Cellular respiration Integral membrane proteins Metabolism Redox
nutrient
A nutrient is a substance used by an organism to survive, grow, and reproduce. The requirement for dietary nutrient intake applies to animals, plants, fungi, and protists. Nutrients can be incorporated into cells for metabolic purposes or excret ...
s, thereby releasing chemical energy in order to produce adenosine triphosphate (ATP). In eukaryotes
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacte ...
, this takes place inside mitochondria
A mitochondrion (; ) is an organelle found in the Cell (biology), cells of most Eukaryotes, such as animals, plants and Fungus, fungi. Mitochondria have a double lipid bilayer, membrane structure and use aerobic respiration to generate adenosi ...
. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is so pervasive because it releases more energy than alternative fermentation
Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food ...
processes such as anaerobic glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvate (). The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH ...
.
The energy stored in the chemical bonds of glucose is released by the cell in the citric acid cycle producing carbon dioxide, and the energetic electron donors NADH and FADH. Oxidative phosphorylation uses these molecules and O2 to produce ATP, which is used throughout the cell whenever energy is needed. During oxidative phosphorylation, electrons are transferred from the electron donors to a series of electron acceptors
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxid ...
in a series of redox reactions ending in oxygen, whose reaction releases half of the total energy.Voet, D.; Voet, J. G. (2004). "Biochemistry", 3rd ed., p. 804, Wiley.ISBN 0-471-19350-X.
In eukaryote
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacte ...
s, these redox reactions are catalyzed by a series of protein complexes within the inner membrane of the cell's mitochondria, whereas, in prokaryotes, these proteins are located in the cell's outer membrane. These linked sets of proteins are called the electron transport chain
An electron transport chain (ETC) is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples th ...
. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.
The energy transferred by electrons flowing through this electron transport chain is used to transport proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s across the inner mitochondrial membrane, in a process called '' electron transport''. This generates potential energy
In physics, potential energy is the energy held by an object because of its position relative to other objects, stresses within itself, its electric charge, or other factors.
Common types of potential energy include the gravitational potentia ...
in the form of a pH gradient and an electrical potential across this membrane. This store of energy is tapped when protons flow back across the membrane and down the potential energy gradient, through a large enzyme called ATP synthase
ATP synthase is a protein that catalyzes the formation of the energy storage molecule adenosine triphosphate (ATP) using adenosine diphosphate (ADP) and inorganic phosphate (Pi). It is classified under ligases as it changes ADP by the formation ...
in a process called chemiosmosis. The ATP synthase uses the energy to transform adenosine diphosphate
Adenosine diphosphate (ADP), also known as adenosine pyrophosphate (APP), is an important organic compound in metabolism and is essential to the flow of energy in living cells. ADP consists of three important structural components: a sugar backbon ...
(ADP) into adenosine triphosphate, in a phosphorylation
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, wh ...
reaction. The reaction is driven by the proton flow, which forces the rotation
Rotation, or spin, is the circular movement of an object around a '' central axis''. A two-dimensional rotating object has only one possible central axis and can rotate in either a clockwise or counterclockwise direction. A three-dimensional ...
of a part of the enzyme. The ATP synthase is a rotary mechanical motor.
Although oxidative phosphorylation is a vital part of metabolism, it produces reactive oxygen species such as superoxide and hydrogen peroxide, which lead to propagation of free radicals
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spont ...
, damaging cells and contributing to disease and, possibly, aging
Ageing ( BE) or aging ( AE) is the process of becoming older. The term refers mainly to humans, many other animals, and fungi, whereas for example, bacteria, perennial plants and some simple animals are potentially biologically immortal. In ...
and senescence
Senescence () or biological aging is the gradual deterioration of functional characteristics in living organisms. The word ''senescence'' can refer to either cellular senescence or to senescence of the whole organism. Organismal senescence inv ...
. The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit their activities.
Chemiosmosis
Oxidative phosphorylation works by using energy-releasing chemical reactions to drive energy-requiring reactions. The two sets of reactions are said to be ''coupled''. This means one cannot occur without the other. The chain of redox reactions driving the flow of electrons through the electron transport chain, from electron donors such as NADH toelectron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process. Electron acceptors are sometimes mista ...
s such as oxygen and hydrogen (protons), is an exergonic process – it releases energy, whereas the synthesis of ATP is an endergonic process, which requires an input of energy. Both the electron transport chain and the ATP synthase are embedded in a membrane, and energy is transferred from the electron transport chain to the ATP synthase by movements of protons across this membrane, in a process called '' chemiosmosis''. A current of protons is driven from the negative N-side of the membrane to the positive P-side through the proton-pumping enzymes of the electron transport chain. The movement of protons creates an electrochemical gradient across the membrane, is called the proton-motive force. It has two components: a difference in proton concentration (a H+ gradient, Δ pH) and a difference in electric potential, with the N-side having a negative charge.
ATP synthase releases this stored energy by completing the circuit and allowing protons to flow down the electrochemical gradient, back to the N-side of the membrane. The electrochemical gradient drives the rotation of part of the enzyme's structure and couples this motion to the synthesis of ATP.
The two components of the proton-motive force are thermodynamically equivalent: In mitochondria, the largest part of energy is provided by the potential; in alkaliphile bacteria the electrical energy even has to compensate for a counteracting inverse pH difference. Inversely, chloroplast
A chloroplast () is a type of membrane-bound organelle known as a plastid that conducts photosynthesis mostly in plant and algal cells. The photosynthetic pigment chlorophyll captures the energy from sunlight, converts it, and stores it in ...
s operate mainly on ΔpH. However, they also require a small membrane potential for the kinetics of ATP synthesis. In the case of the fusobacterium '' Propionigenium modestum'' it drives the counter-rotation of subunits a and c of the FO motor of ATP synthase.
The amount of energy released by oxidative phosphorylation is high, compared with the amount produced by anaerobic fermentation. Glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvate (). The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH ...
produces only 2 ATP molecules, but somewhere between 30 and 36 ATPs are produced by the oxidative phosphorylation of the 10 NADH and 2 succinate molecules made by converting one molecule of glucose to carbon dioxide and water, while each cycle of beta oxidation of a fatty acid yields about 14 ATPs. These ATP yields are theoretical maximum values; in practice, some protons leak across the membrane, lowering the yield of ATP.
Electron and proton transfer molecules
cytochrome c
The cytochrome complex, or cyt ''c'', is a small hemeprotein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins and plays a major role in cell apoptosis. Cytochrome c is hig ...
. This carries only electrons, and these are transferred by the reduction and oxidation of an iron atom that the protein holds within a heme group in its structure. Cytochrome c is also found in some bacteria, where it is located within the periplasmic space.
Within the inner mitochondrial membrane, the lipid-soluble electron carrier coenzyme Q10
Coenzyme Q, also known as ubiquinone and marketed as CoQ10, is a coenzyme family that is ubiquitous in animals and most bacteria (hence the name ubiquinone). In humans, the most common form is coenzyme Q10 or ubiquinone-10.
It is a 1,4-benzoq ...
(Q) carries both electrons and protons by a redox cycle. This small benzoquinone molecule is very hydrophobic, so it diffuses freely within the membrane. When Q accepts two electrons and two protons, it becomes reduced to the '' ubiquinol'' form (QH2); when QH2 releases two electrons and two protons, it becomes oxidized back to the ''ubiquinone'' (Q) form. As a result, if two enzymes are arranged so that Q is reduced on one side of the membrane and QH2 oxidized on the other, ubiquinone will couple these reactions and shuttle protons across the membrane. Some bacterial electron transport chains use different quinones, such as menaquinone, in addition to ubiquinone.
Within proteins, electrons are transferred between flavin cofactors, iron–sulfur clusters and cytochromes. There are several types of iron–sulfur cluster. The simplest kind found in the electron transfer chain consists of two iron atoms joined by two atoms of inorganic sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
; these are called Fe–2Sclusters. The second kind, called Fe–4S contains a cube of four iron atoms and four sulfur atoms. Each iron atom in these clusters is coordinated by an additional amino acid, usually by the sulfur atom of cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, sometime ...
. Metal ion cofactors undergo redox reactions without binding or releasing protons, so in the electron transport chain they serve solely to transport electrons through proteins. Electrons move quite long distances through proteins by hopping along chains of these cofactors. This occurs by quantum tunnelling
Quantum tunnelling, also known as tunneling ( US) is a quantum mechanical phenomenon whereby a wavefunction can propagate through a potential barrier.
The transmission through the barrier can be finite and depends exponentially on the barrier h ...
, which is rapid over distances of less than 1.4 m.
Eukaryotic electron transport chains
Manycatabolic
Catabolism () is the set of metabolic pathways that breaks down molecules into smaller units that are either oxidized to release energy or used in other anabolic reactions. Catabolism breaks down large molecules (such as polysaccharides, lipids, ...
biochemical processes, such as glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvate (). The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH ...
, the citric acid cycle, and beta oxidation, produce the reduced coenzyme NADH. This coenzyme contains electrons that have a high transfer potential; in other words, they will release a large amount of energy upon oxidation. However, the cell does not release this energy all at once, as this would be an uncontrollable reaction. Instead, the electrons are removed from NADH and passed to oxygen through a series of enzymes that each release a small amount of the energy. This set of enzymes, consisting of complexes I through IV, is called the electron transport chain and is found in the inner membrane of the mitochondrion. Succinate is also oxidized by the electron transport chain, but feeds into the pathway at a different point.
In eukaryote
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacte ...
s, the enzymes in this electron transport system use the energy released from O2 by NADH to pump proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s across the inner membrane of the mitochondrion. This causes protons to build up in the intermembrane space, and generates an electrochemical gradient across the membrane. The energy stored in this potential is then used by ATP synthase to produce ATP. Oxidative phosphorylation in the eukaryotic mitochondrion is the best-understood example of this process. The mitochondrion is present in almost all eukaryotes, with the exception of anaerobic protozoa such as '' Trichomonas vaginalis'' that instead reduce protons to hydrogen in a remnant mitochondrion called a hydrogenosome.
NADH-coenzyme Q oxidoreductase (complex I)
kilodaltons
The dalton or unified atomic mass unit (symbols: Da or u) is a non-SI unit of mass widely used in physics and chemistry. It is defined as of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at ...
(kDa). The structure is known in detail only from a bacterium; in most organisms the complex resembles a boot with a large "ball" poking out from the membrane into the mitochondrion. The genes that encode the individual proteins are contained in both the cell nucleus
The cell nucleus (pl. nuclei; from Latin or , meaning ''kernel'' or ''seed'') is a membrane-bound organelle found in eukaryotic cells. Eukaryotic cells usually have a single nucleus, but a few cell types, such as mammalian red blood cells, h ...
and the mitochondrial genome, as is the case for many enzymes present in the mitochondrion.
The reaction that is catalyzed by this enzyme is the two electron oxidation of NADH by coenzyme Q10
Coenzyme Q, also known as ubiquinone and marketed as CoQ10, is a coenzyme family that is ubiquitous in animals and most bacteria (hence the name ubiquinone). In humans, the most common form is coenzyme Q10 or ubiquinone-10.
It is a 1,4-benzoq ...
or ''ubiquinone'' (represented as Q in the equation below), a lipid-soluble quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds
that is found in the mitochondrion membrane:
The start of the reaction, and indeed of the entire electron chain, is the binding of a NADH molecule to complex I and the donation of two electrons. The electrons enter complex I via a prosthetic group attached to the complex, flavin mononucleotide (FMN). The addition of electrons to FMN converts it to its reduced form, FMNH2. The electrons are then transferred through a series of iron–sulfur clusters: the second kind of prosthetic group present in the complex. There are both Fe–2Sand Fe–4Siron–sulfur clusters in complex I.
As the electrons pass through this complex, four protons are pumped from the matrix into the intermembrane space. Exactly how this occurs is unclear, but it seems to involve uch as benzene or naphthalene
Uch ( pa, ;
ur, ), frequently referred to as Uch Sharīf ( pa, ;
ur, ; ''"Noble Uch"''), is a historic city in the southern part of Pakistan's Punjab province. Uch may have been founded as Alexandria on the Indus, a town founded by Alexand ...
by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double ...conformational change
In biochemistry, a conformational change is a change in the shape of a macromolecule, often induced by environmental factors.
A macromolecule is usually flexible and dynamic. Its shape can change in response to changes in its environment or oth ...
s in complex I that cause the protein to bind protons on the N-side of the membrane and release them on the P-side of the membrane. Finally, the electrons are transferred from the chain of iron–sulfur clusters to a ubiquinone molecule in the membrane. Reduction of ubiquinone also contributes to the generation of a proton gradient, as two protons are taken up from the matrix as it is reduced to ubiquinol (QH2).
Succinate-Q oxidoreductase (complex II)
flavin adenine dinucleotide
Flavin may refer to:
Placename
* Flavin, Aveyron, a commune in southern France
Surname
* Adrian Flavin (born 1979), a professional rugby player
* Christopher Flavin, president of the Worldwatch Institute
* Dan Flavin (1933–1996), a minimalis ...
(FAD) cofactor, iron–sulfur clusters, and a heme group that does not participate in electron transfer to coenzyme Q, but is believed to be important in decreasing production of reactive oxygen species. It oxidizes succinate to fumarate
Fumaric acid is an organic compound with the formula HO2CCH=CHCO2H. A white solid, fumaric acid occurs widely in nature. It has a fruit-like taste and has been used as a food additive. Its E number is E297.
The salts and esters are known as f ...
and reduces ubiquinone. As this reaction releases less energy than the oxidation of NADH, complex II does not transport protons across the membrane and does not contribute to the proton gradient.
In some eukaryotes, such as the parasitic worm '' Ascaris suum'', an enzyme similar to complex II, fumarate reductase (menaquinol:fumarate
oxidoreductase, or QFR), operates in reverse to oxidize ubiquinol and reduce fumarate. This allows the worm to survive in the anaerobic environment of the large intestine, carrying out anaerobic oxidative phosphorylation with fumarate as the electron acceptor. Another unconventional function of complex II is seen in the malaria parasite ''Plasmodium falciparum
''Plasmodium falciparum'' is a Unicellular organism, unicellular protozoan parasite of humans, and the deadliest species of ''Plasmodium'' that causes malaria in humans. The parasite is transmitted through the bite of a female ''Anopheles'' mosqu ...
''. Here, the reversed action of complex II as an oxidase is important in regenerating ubiquinol, which the parasite uses in an unusual form of pyrimidine
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The other ...
biosynthesis.
Electron transfer flavoprotein-Q oxidoreductase
Electron transfer flavoprotein-ubiquinone oxidoreductase (ETF-Q oxidoreductase), also known as ''electron transferring-flavoprotein dehydrogenase'', is a third entry point to the electron transport chain. It is an enzyme that accepts electrons from electron-transferring flavoprotein in the mitochondrial matrix, and uses these electrons to reduce ubiquinone. This enzyme contains a flavin and a Fe–4Scluster, but, unlike the other respiratory complexes, it attaches to the surface of the membrane and does not cross the lipid bilayer. In mammals, this metabolic pathway is important in beta oxidation of fatty acids and catabolism of amino acids and choline, as it accepts electrons from multipleacetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for ...
dehydrogenases. In plants, ETF-Q oxidoreductase is also important in the metabolic responses that allow survival in extended periods of darkness.
Q-cytochrome c oxidoreductase (complex III)
dimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* Julius Dimer ( ...
, with each subunit complex containing 11 protein subunits, an Fe-2Siron–sulfur cluster and three cytochromes: one cytochrome c1 and two b cytochromes. A cytochrome is a kind of electron-transferring protein that contains at least one heme group. The iron atoms inside complex III's heme groups alternate between a reduced ferrous (+2) and oxidized ferric (+3) state as the electrons are transferred through the protein.
The reaction catalyzed by complex III is the oxidation of one molecule of ubiquinol and the reduction of two molecules of cytochrome c
The cytochrome complex, or cyt ''c'', is a small hemeprotein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins and plays a major role in cell apoptosis. Cytochrome c is hig ...
, a heme protein loosely associated with the mitochondrion. Unlike coenzyme Q, which carries two electrons, cytochrome c carries only one electron.
As only one of the electrons can be transferred from the QH2 donor to a cytochrome c acceptor at a time, the reaction mechanism of complex III is more elaborate than those of the other respiratory complexes, and occurs in two steps called the Q cycle
The Q cycle (named for ''quinol'') describes a series of reactions that describe how the sequential oxidation and reduction of the lipophilic electron carrier, Coenzyme Q (CoQ), between the ubiquinol and ubiquinone forms, can result in the net move ...
. In the first step, the enzyme binds three substrates, first, QH2, which is then oxidized, with one electron being passed to the second substrate, cytochrome c. The two protons released from QH2 pass into the intermembrane space. The third substrate is Q, which accepts the second electron from the QH2 and is reduced to Q.−, which is the ubisemiquinone free radical. The first two substrates are released, but this ubisemiquinone intermediate remains bound. In the second step, a second molecule of QH2 is bound and again passes its first electron to a cytochrome c acceptor. The second electron is passed to the bound ubisemiquinone, reducing it to QH2 as it gains two protons from the mitochondrial matrix. This QH2 is then released from the enzyme.
As coenzyme Q is reduced to ubiquinol on the inner side of the membrane and oxidized to ubiquinone on the other, a net transfer of protons across the membrane occurs, adding to the proton gradient. The rather complex two-step mechanism by which this occurs is important, as it increases the efficiency of proton transfer. If, instead of the Q cycle, one molecule of QH2 were used to directly reduce two molecules of cytochrome c, the efficiency would be halved, with only one proton transferred per cytochrome c reduced.
Cytochrome c oxidase (complex IV)
electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process. Electron acceptors are sometimes mista ...
oxygen is reduced to water in this step. Both the direct pumping of protons and the consumption of matrix protons in the reduction of oxygen contribute to the proton gradient. The reaction catalyzed is the oxidation of cytochrome c and the reduction of oxygen:
Alternative reductases and oxidases
Many eukaryotic organisms have electron transport chains that differ from the much-studied mammalian enzymes described above. For example, plants have alternative NADH oxidases, which oxidize NADH in the cytosol rather than in the mitochondrial matrix, and pass these electrons to the ubiquinone pool. These enzymes do not transport protons, and, therefore, reduce ubiquinone without altering the electrochemical gradient across the inner membrane. Another example of a divergent electron transport chain is the ''alternative oxidase
Alternative or alternate may refer to:
Arts, entertainment and media
* Alternative (''Kamen Rider''), a character in the Japanese TV series ''Kamen Rider Ryuki''
* ''The Alternative'' (film), a 1978 Australian television film
* ''The Alternative ...
'', which is found in plants, as well as some fungi, protists, and possibly some animals. This enzyme transfers electrons directly from ubiquinol to oxygen.
The electron transport pathways produced by these alternative NADH and ubiquinone oxidases have lower ATP
ATP may refer to:
Companies and organizations
* Association of Tennis Professionals, men's professional tennis governing body
* American Technical Publishers, employee-owned publishing company
* ', a Danish pension
* Armenia Tree Project, non ...
yields than the full pathway. The advantages produced by a shortened pathway are not entirely clear. However, the alternative oxidase is produced in response to stresses such as cold, reactive oxygen species, and infection by pathogens, as well as other factors that inhibit the full electron transport chain. Alternative pathways might, therefore, enhance an organisms' resistance to injury, by reducing oxidative stress.
Organization of complexes
The original model for how the respiratory chain complexes are organized was that they diffuse freely and independently in the mitochondrial membrane. However, recent data suggest that the complexes might form higher-order structures called supercomplexes or "respirasome
Modern biological research has revealed strong evidence that the enzymes of the mitochondrial respiratory chain assemble into larger, supramolecular structures called supercomplexes, instead of the traditional fluid model of discrete enzymes dispe ...
s". In this model, the various complexes exist as organized sets of interacting enzymes. These associations might allow channeling of substrates between the various enzyme complexes, increasing the rate and efficiency of electron transfer. Within such mammalian supercomplexes, some components would be present in higher amounts than others, with some data suggesting a ratio between complexes I/II/III/IV and the ATP synthase of approximately 1:1:3:7:4. However, the debate over this supercomplex hypothesis is not completely resolved, as some data do not appear to fit with this model.
Prokaryotic electron transport chains
In contrast to the general similarity in structure and function of the electron transport chains in eukaryotes, bacteria andarchaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria (in the Archaebac ...
possess a large variety of electron-transfer enzymes. These use an equally wide set of chemicals as substrates. In common with eukaryotes, prokaryotic electron transport uses the energy released from the oxidation of a substrate to pump ions across a membrane and generate an electrochemical gradient. In the bacteria, oxidative phosphorylation in '' Escherichia coli'' is understood in most detail, while archaeal systems are at present poorly understood.
The main difference between eukaryotic and prokaryotic oxidative phosphorylation is that bacteria and archaea use many different substances to donate or accept electrons. This allows prokaryotes to grow under a wide variety of environmental conditions. In ''E. coli'', for example, oxidative phosphorylation can be driven by a large number of pairs of reducing agents and oxidizing agents, which are listed below. The midpoint potential of a chemical measures how much energy is released when it is oxidized or reduced, with reducing agents having negative potentials and oxidizing agents positive potentials.
As shown above, ''E. coli'' can grow with reducing agents such as formate, hydrogen, or lactate as electron donors, and nitrate, DMSO, or oxygen as acceptors. The larger the difference in midpoint potential between an oxidizing and reducing agent, the more energy is released when they react. Out of these compounds, the succinate/fumarate pair is unusual, as its midpoint potential is close to zero. Succinate can therefore be oxidized to fumarate if a strong oxidizing agent such as oxygen is available, or fumarate can be reduced to succinate using a strong reducing agent such as formate. These alternative reactions are catalyzed by succinate dehydrogenase
Succinate dehydrogenase (SDH) or succinate-coenzyme Q reductase (SQR) or respiratory complex II is an enzyme complex, found in many bacterial cells and in the inner mitochondrial membrane of eukaryotes. It is the only enzyme that participates i ...
and fumarate reductase, respectively.
Some prokaryotes use redox pairs that have only a small difference in midpoint potential. For example, nitrifying bacteria such as '' Nitrobacter'' oxidize nitrite to nitrate, donating the electrons to oxygen. The small amount of energy released in this reaction is enough to pump protons and generate ATP, but not enough to produce NADH or NADPH directly for use in anabolism
Anabolism () is the set of metabolic pathways that construct molecules from smaller units. These reactions require energy, known also as an endergonic process. Anabolism is the building-up aspect of metabolism, whereas catabolism is the breaking-do ...
. This problem is solved by using a nitrite oxidoreductase
Nitrite oxidoreductase (NOR or NXR) is an enzyme involved in nitrification. It is the last step in the process of aerobic ammonia oxidation, which is carried out by two groups of nitrifying bacteria: ammonia oxidizers such as '' Nitrosospira'', ''N ...
to produce enough proton-motive force to run part of the electron transport chain in reverse, causing complex I to generate NADH.
Prokaryotes control their use of these electron donors and acceptors by varying which enzymes are produced, in response to environmental conditions. This flexibility is possible because different oxidases and reductases use the same ubiquinone pool. This allows many combinations of enzymes to function together, linked by the common ubiquinol intermediate. These respiratory chains therefore have a modular design
Modular design, or modularity in design, is a design principle that subdivides a system into smaller parts called ''modules'' (such as modular process skids), which can be independently created, modified, replaced, or exchanged with other modules ...
, with easily interchangeable sets of enzyme systems.
In addition to this metabolic diversity, prokaryotes also possess a range of isozymes – different enzymes that catalyze the same reaction. For example, in ''E. coli'', there are two different types of ubiquinol oxidase using oxygen as an electron acceptor. Under highly aerobic conditions, the cell uses an oxidase with a low affinity for oxygen that can transport two protons per electron. However, if levels of oxygen fall, they switch to an oxidase that transfers only one proton per electron, but has a high affinity for oxygen.
ATP synthase (complex V)
ATP synthase, also called ''complex V'', is the final enzyme in the oxidative phosphorylation pathway. This enzyme is found in all forms of life and functions in the same way in both prokaryotes and eukaryotes. The enzyme uses the energy stored in a proton gradient across a membrane to drive the synthesis of ATP from ADP and phosphate (Pi). Estimates of the number of protons required to synthesize one ATP have ranged from three to four, with some suggesting cells can vary this ratio, to suit different conditions. Thisphosphorylation
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, wh ...
reaction is an equilibrium, which can be shifted by altering the proton-motive force. In the absence of a proton-motive force, the ATP synthase reaction will run from right to left, hydrolyzing ATP and pumping protons out of the matrix across the membrane. However, when the proton-motive force is high, the reaction is forced to run in the opposite direction; it proceeds from left to right, allowing protons to flow down their concentration gradient and turning ADP into ATP. Indeed, in the closely related vacuolar type H+-ATPases, the hydrolysis reaction is used to acidify cellular compartments, by pumping protons and hydrolysing ATP.
ATP synthase is a massive protein complex with a mushroom-like shape. The mammalian enzyme complex contains 16 subunits and has a mass of approximately 600 kilodaltons. The portion embedded within the membrane is called FO and contains a ring of c subunits and the proton channel. The stalk and the ball-shaped headpiece is called F1 and is the site of ATP synthesis. The ball-shaped complex at the end of the F1 portion contains six proteins of two different kinds (three α subunits and three β subunits), whereas the "stalk" consists of one protein: the γ subunit, with the tip of the stalk extending into the ball of α and β subunits. Both the α and β subunits bind nucleotides, but only the β subunits catalyze the ATP synthesis reaction. Reaching along the side of the F1 portion and back into the membrane is a long rod-like subunit that anchors the α and β subunits into the base of the enzyme.
As protons cross the membrane through the channel in the base of ATP synthase, the FO proton-driven motor rotates. Rotation might be caused by changes in the ionization of amino acids in the ring of c subunits causing electrostatic interactions that propel the ring of c subunits past the proton channel. This rotating ring in turn drives the rotation of the central axle
An axle or axletree is a central shaft for a rotating wheel or gear. On wheeled vehicles, the axle may be fixed to the wheels, rotating with them, or fixed to the vehicle, with the wheels rotating around the axle. In the former case, bearing ...
(the γ subunit stalk) within the α and β subunits. The α and β subunits are prevented from rotating themselves by the side-arm, which acts as a stator
The stator is the stationary part of a rotary system, found in electric generators, electric motors, sirens, mud motors or biological rotors. Energy flows through a stator to or from the rotating component of the system. In an electric mot ...
. This movement of the tip of the γ subunit within the ball of α and β subunits provides the energy for the active sites in the β subunits to undergo a cycle of movements that produces and then releases ATP.
 This ATP synthesis reaction is called the ''binding change mechanism'' and involves the active site of a β subunit cycling between three states. In the "open" state, ADP and phosphate enter the active site (shown in brown in the diagram). The protein then closes up around the molecules and binds them loosely – the "loose" state (shown in red). The enzyme then changes shape again and forces these molecules together, with the active site in the resulting "tight" state (shown in pink) binding the newly produced ATP molecule with very high
This ATP synthesis reaction is called the ''binding change mechanism'' and involves the active site of a β subunit cycling between three states. In the "open" state, ADP and phosphate enter the active site (shown in brown in the diagram). The protein then closes up around the molecules and binds them loosely – the "loose" state (shown in red). The enzyme then changes shape again and forces these molecules together, with the active site in the resulting "tight" state (shown in pink) binding the newly produced ATP molecule with very high affinity
Affinity may refer to:
Commerce, finance and law
* Affinity (law), kinship by marriage
* Affinity analysis, a market research and business management technique
* Affinity Credit Union, a Saskatchewan-based credit union
* Affinity Equity Partn ...
. Finally, the active site cycles back to the open state, releasing ATP and binding more ADP and phosphate, ready for the next cycle.
In some bacteria and archaea, ATP synthesis is driven by the movement of sodium ions through the cell membrane, rather than the movement of protons. Archaea such as ''Methanococcus
''Methanococcus'' is a genus of coccoid methanogens of the family Methanococcaceae. They are all mesophiles, except the thermophilic '' M. thermolithotrophicus'' and the hyperthermophilic '' M. jannaschii''. The latter was discovered at the base ...
'' also contain the A1Ao synthase, a form of the enzyme that contains additional proteins with little similarity in sequence to other bacterial and eukaryotic ATP synthase subunits. It is possible that, in some species, the A1Ao form of the enzyme is a specialized sodium-driven ATP synthase, but this might not be true in all cases.
Oxidative phosphorylation - energetics
The transport of electrons from redox pair NAD+/ NADH to the final redox pair 1/2 O2/ H2O can be summarized as 1/2 O2 + NADH + H+ → H2O + NAD+ The potential difference between these two redox pairs is 1.14 volt, which is equivalent to -52 kcal/mol or -2600 kJ per 6 mol of O2. When one NADH is oxidized through the electron transfer chain, three ATPs are produced, which is equivalent to 7.3 kcal/mol x 3 = 21.9 kcal/mol. The conservation of the energy can be calculated by the following formula Efficiency = (21.9 x 100%) / 52 = 42% So we can conclude that when NADH is oxidized, about 42% of energy is conserved in the form of three ATPs and the remaining (58%) energy is lost as heat (unless the chemical energy of ATP under physiological conditions was underestimated).Reactive oxygen species
Molecular oxygen is a good terminalelectron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process. Electron acceptors are sometimes mista ...
because it is a strong oxidizing agent. The reduction of oxygen does involve potentially harmful intermediates. Although the transfer of four electrons and four protons reduces oxygen to water, which is harmless, transfer of one or two electrons produces superoxide or peroxide anions, which are dangerously reactive.
These reactive oxygen species and their reaction products, such as the hydroxyl radical, are very harmful to cells, as they oxidize proteins and cause mutations in DNA. This cellular damage might contribute to disease and is proposed as one cause of aging
Ageing ( BE) or aging ( AE) is the process of becoming older. The term refers mainly to humans, many other animals, and fungi, whereas for example, bacteria, perennial plants and some simple animals are potentially biologically immortal. In ...
.
The cytochrome c oxidase complex is highly efficient at reducing oxygen to water, and it releases very few partly reduced intermediates; however small amounts of superoxide anion and peroxide are produced by the electron transport chain. Particularly important is the reduction of coenzyme Q
Coenzyme Q, also known as ubiquinone and marketed as CoQ10, is a coenzyme family that is ubiquitous in animals and most bacteria (hence the name ubiquinone). In humans, the most common form is coenzyme Q10 or ubiquinone-10.
It is a 1,4-benzoq ...
in complex III, as a highly reactive ubisemiquinone free radical is formed as an intermediate in the Q cycle. This unstable species can lead to electron "leakage" when electrons transfer directly to oxygen, forming superoxide. As the production of reactive oxygen species by these proton-pumping complexes is greatest at high membrane potentials, it has been proposed that mitochondria regulate their activity to maintain the membrane potential within a narrow range that balances ATP production against oxidant generation. For instance, oxidants can activate uncoupling proteins that reduce membrane potential.
To counteract these reactive oxygen species, cells contain numerous antioxidant
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. This can lead to polymerization and other chain reactions. They are frequently added to industrial products, such as fuels and lubricant ...
systems, including antioxidant vitamins such as vitamin C and vitamin E, and antioxidant enzymes such as superoxide dismutase
Superoxide dismutase (SOD, ) is an enzyme that alternately catalyzes the dismutation (or partitioning) of the superoxide () radical into ordinary molecular oxygen (O2) and hydrogen peroxide (). Superoxide is produced as a by-product of oxygen me ...
, catalase
Catalase is a common enzyme found in nearly all living organisms exposed to oxygen (such as bacteria, plants, and animals) which catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is a very important enzyme in protecting t ...
, and peroxidases, which detoxify the reactive species, limiting damage to the cell.
Oxidative phosphorylation in hypoxic conditions
As oxygen is fundamental for oxidative phosphorylation, a shortage in O2 level likely alters ATP production rates. However, proton motive force and ATP production can be maintained by intracellular acidosis. Cytosolic protons that have accumulated with ATP hydrolysis and lactic acidosis can freely diffuse across the mitochondrial outer-membrane and acidify the inter-membrane space, hence directly contributing to the proton motive force and ATP production.Inhibitors
There are several well-knowndrug
A drug is any chemical substance that causes a change in an organism's physiology or psychology when consumed. Drugs are typically distinguished from food and substances that provide nutritional support. Consumption of drugs can be via insuffla ...
s and toxins that inhibit oxidative phosphorylation. Although any one of these toxins inhibits only one enzyme in the electron transport chain, inhibition of any step in this process will halt the rest of the process. For example, if oligomycin inhibits ATP synthase, protons cannot pass back into the mitochondrion. As a result, the proton pumps are unable to operate, as the gradient becomes too strong for them to overcome. NADH is then no longer oxidized and the citric acid cycle ceases to operate because the concentration of NAD+ falls below the concentration that these enzymes can use.
Many site-specific inhibitors of the electron transport chain have contributed to the present knowledge of mitochondrial respiration. Synthesis of ATP is also dependent on the electron transport chain, so all site-specific inhibitors also inhibit ATP formation. The fish poison rotenone, the barbiturate drug amytal
Amobarbital (formerly known as amylobarbitone or sodium amytal as the soluble sodium salt) is a drug that is a barbiturate derivative. It has sedative-hypnotic properties. It is a white crystalline powder with no odor and a slightly bitter taste. ...
, and the antibiotic piericidin A
Piericidin A is an antibiotic agent. It was discovered from '' Streptomyces mobaraensis''. Being an inhibitor of NADH dehydrogenase, it inhibits electron transfer; its structure resembles that of the ubiquinone, therefore it competes with QB for bi ...
inhibit NADH and coenzyme Q.
Carbon monoxide, cyanide, hydrogen sulphide and azide effectively inhibit cytochrome oxidase. Carbon monoxide reacts with the reduced form of the cytochrome while cyanide and azide react with the oxidised form. An antibiotic, antimycin A, and British anti-Lewisite
Dimercaprol, also called British anti-Lewisite (BAL), is a medication used to treat acute poisoning by arsenic, mercury, gold, and lead. It may also be used for antimony, thallium, or bismuth poisoning, although the evidence for those uses is not ...
, an antidote used against chemical weapons, are the two important inhibitors of the site between cytochrome B and C1.
Not all inhibitors of oxidative phosphorylation are toxins. In brown adipose tissue, regulated proton channels called uncoupling proteins can uncouple respiration from ATP synthesis. This rapid respiration produces heat, and is particularly important as a way of maintaining body temperature for hibernating
Hibernation is a state of minimal activity and metabolic depression undergone by some animal species. Hibernation is a seasonal heterothermy characterized by low body-temperature, slow breathing and heart-rate, and low metabolic rate. It most ...
animals, although these proteins may also have a more general function in cells' responses to stress.
History
The field of oxidative phosphorylation began with the report in 1906 by Arthur Harden of a vital role for phosphate in cellularfermentation
Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food ...
, but initially only sugar phosphates
Sugar phosphates (sugars that have added or substituted phosphate groups) are often used in biological systems to store or transfer energy. They also form the backbone for DNA and RNA. Sugar phosphate backbone geometry is altered in the vicinity ...
were known to be involved. However, in the early 1940s, the link between the oxidation of sugars and the generation of ATP was firmly established by Herman Kalckar
Herman Moritz Kalckar (26 March 1908 – 17 May 1991) was a Danish biochemist who pioneered the study of cellular respiration. Kalckar made a number of significant contributions to the development of 20th century biochemistry including:
* a founder ...
, confirming the central role of ATP in energy transfer that had been proposed by Fritz Albert Lipmann
Fritz Albert Lipmann (; June 12, 1899 – July 24, 1986) was a German-American biochemist and a co-discoverer in 1945 of coenzyme A. For this, together with other research on coenzyme A, he was awarded the Nobel Prize in Physiology or Medicine in ...
in 1941. Later, in 1949, Morris Friedkin and Albert L. Lehninger
Albert Lester Lehninger (February 17, 1917 – March 4, 1986) was an American biochemist in the field of bioenergetics. He made fundamental contributions to the current understanding of metabolism at a molecular level. In 1948, he discovered, wit ...
proved that the coenzyme NADH linked metabolic pathways such as the citric acid cycle and the synthesis of ATP. The term ''oxidative phosphorylation'' was coined by in 1939.
For another twenty years, the mechanism by which ATP is generated remained mysterious, with scientists searching for an elusive "high-energy intermediate" that would link oxidation and phosphorylation reactions. This puzzle was solved by Peter D. Mitchell
Peter Dennis Mitchell, FRS (29 September 1920 – 10 April 1992) was a British biochemist who was awarded the 1978 Nobel Prize for Chemistry for his discovery of the chemiosmotic mechanism of ATP synthesis.
Education and early life
Mitc ...
with the publication of the chemiosmotic theory
Chemiosmosis is the movement of ions across a semipermeable membrane bound structure, down their electrochemical gradient. An important example is the formation of adenosine triphosphate (ATP) by the movement of hydrogen ions (H+) across a membra ...
in 1961. At first, this proposal was highly controversial, but it was slowly accepted and Mitchell was awarded a Nobel prize in 1978. Subsequent research concentrated on purifying and characterizing the enzymes involved, with major contributions being made by David E. Green
David Ezra Green (August 5, 1910 – July 8, 1983) was an American biochemist who made significant contributions to the study of enzymes, particularly the electron transport chain and oxidative phosphorylation.
Life and career
Green was born i ...
on the complexes of the electron-transport chain, as well as Efraim Racker
Efraim Racker (June 28, 1913 – September 9, 1991) was an Austrian biochemist who was responsible for identifying and purifying Factor 1 (F1), the first part of the ATP synthase enzyme to be characterised. F1 is only a part of a larger ATP syn ...
on the ATP synthase. A critical step towards solving the mechanism of the ATP synthase was provided by Paul D. Boyer
Paul Delos Boyer (July 31, 1918 – June 2, 2018) was an American biochemist, analytical chemist, and a professor of chemistry at University of California Los Angeles (UCLA). He shared the 1997 Nobel Prize in Chemistry for research on the " enzy ...
, by his development in 1973 of the "binding change" mechanism, followed by his radical proposal of rotational catalysis in 1982. More recent work has included structural studies on the enzymes involved in oxidative phosphorylation by John E. Walker
Sir John Ernest Walker One or more of the preceding sentences incorporates text from the royalsociety.org website where: (born 7 January 1941) is a British chemist who won the Nobel Prize in Chemistry in 1997. Walker is Emeritus Director an ...
, with Walker and Boyer being awarded a Nobel Prize in 1997.
See also
*Respirometry
Respirometry is a general term that encompasses a number of techniques for obtaining estimates of the rates of metabolism of vertebrates, invertebrates, plants, tissues, cells, or microorganisms via an indirect measure of heat production (calorime ...
*TIM/TOM Complex
The TIM/TOM complex is a protein complex in cellular biochemistry which translocates proteins produced from nuclear DNA through the mitochondrial membrane for use in oxidative phosphorylation. In enzymology, the complex is described as an mit ...
Notes
References
Further reading
Introductory
* * *Advanced
* * * *General resources
Animated diagrams illustrating oxidative phosphorylation
Wiley and Co ''Concepts in Biochemistry''
On-line biophysics lectures
Antony Crofts, University of Illinois at Urbana–Champaign
ATP Synthase
Graham Johnson
Structural resources
* PDB molecule of the month:ATP synthase
*Interactive molecular models at Universidade Fernando Pessoa:
NADH dehydrogenase
{{DEFAULTSORT:Oxidative Phosphorylation Cellular respiration Integral membrane proteins Metabolism Redox