|
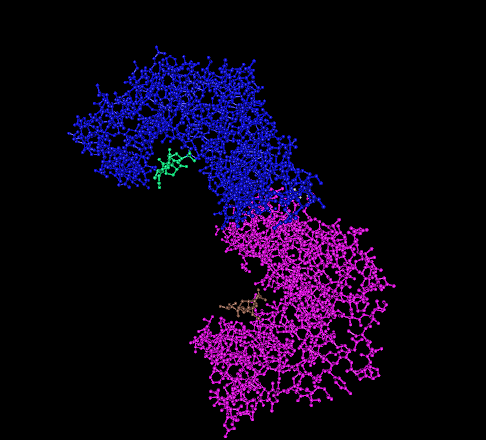
Protein Dimer
In biochemistry, a protein dimer is a macromolecular complex formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", ''di-'' + '' -mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins. A protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein-coupled cannabinoid receptors have the ability to form both homo- and heterodimers with sev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
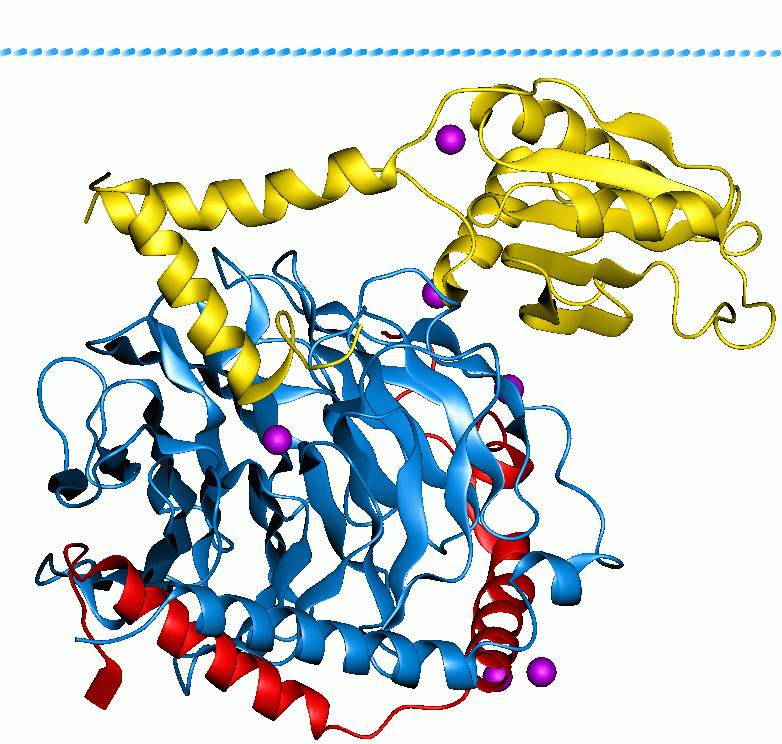
Galactose-1-phosphate Uridylyltransferase 1GUP
D-Galactose-1-phosphate is an intermediate in the intraconversion of glucose and uridine diphosphate galactose. It is formed from galactose by galactokinase.The improper metabolism of galactose-1-phosphate is a characteristic of galactosemia. The Leloir pathway is responsible for such metabolism of galactose and its intermediate, galactose-1-phosphate. Deficiency of enzymes found in this pathway can result in galactosemia; therefore, diagnosis of this genetic disorder occasionally involves measuring the concentration of these enzymes. One of such enzymes is galactose-1-phosphate uridylytransferase (GALT). The enzyme catalyzes the transfer of a UDP-activator group from UDP-glucose to galactose-1-phosphate. Although the cause of enzyme deficiency in the Leloir pathway is still disputed amongst researchers, some studies suggest that protein misfolding of GALT, which may lead to an unfavorable conformational change that impacts its thermal stability and substrate-binding affinity, may p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine Receptor
Dopamine receptors are a class of G protein-coupled receptors that are prominent in the vertebrate central nervous system (CNS). Dopamine receptors activate different effectors through not only G-protein coupling, but also signaling through different protein (dopamine receptor-interacting proteins) interactions. The neurotransmitter dopamine is the primary endogenous ligand for dopamine receptors. Dopamine receptors are implicated in many neurological processes, including motivational and incentive salience, cognition, memory, learning, and fine motor control, as well as modulation of neuroendocrine signaling. Abnormal dopamine receptor signaling and dopaminergic nerve function is implicated in several neuropsychiatric disorders. Thus, dopamine receptors are common neurologic drug targets; antipsychotics are often dopamine receptor antagonists while psychostimulants are typically indirect agonists of dopamine receptors. Subtypes The existence of multiple types of receptors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Protein
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases. There are two classes of G proteins. The first function as monomeric small GTPases (small G-proteins), while the second function as heterotrimeric G protein complexes. The latter class of complexes is made up of ''alpha'' (α), ''beta'' (β) and ''gamma'' (γ) subunits. In addition, the beta and gamma subunits can form a stable dimeric complex referred to as the beta-gamma complex . Heterotrimeric G proteins located within the cell a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Receptor
In the field of molecular biology, nuclear receptors are a class of proteins responsible for sensing steroids, thyroid hormones, vitamins, and certain other molecules. These receptors work with other proteins to regulate the expression of specific genes thereby controlling the development, homeostasis, and metabolism of the organism. Nuclear receptors bind directly to DNA regulating the expression of adjacent genes; hence these receptors are classified as transcription factors. The regulation of gene expression by nuclear receptors often occurs in the presence of a ligand—a molecule that affects the receptor's behavior. Ligand binding to a nuclear receptor results in a conformational change activating the receptor. The result is up- or down-regulation of gene expression. A unique property of nuclear receptors that differentiates them from other classes of receptors is their direct control of genomic DNA. Nuclear receptors play key roles in both embryonic deve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor (biochemistry)
In biochemistry and pharmacology, receptors are chemical structures, composed of protein, that receive and transduce signals that may be integrated into biological systems. These signals are typically chemical messengers which bind to a receptor and cause some form of cellular/tissue response, e.g. a change in the electrical activity of a cell. There are three main ways the action of the receptor can be classified: relay of signal, amplification, or integration. Relaying sends the signal onward, amplification increases the effect of a single ligand, and integration allows the signal to be incorporated into another biochemical pathway. Receptor proteins can be classified by their location. Transmembrane receptors include ligand-gated ion channels, G protein-coupled receptors, and enzyme-linked hormone receptors. Intracellular receptors are those found inside the cell, and include cytoplasmic receptors and nuclear receptors. A molecule that binds to a receptor is called a li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fibrin
Fibrin (also called Factor Ia) is a fibrous, non-globular protein involved in the clotting of blood. It is formed by the action of the protease thrombin on fibrinogen, which causes it to polymerize. The polymerized fibrin, together with platelets, forms a hemostatic plug or clot over a wound site. When the lining of a blood vessel is broken, platelets are attracted, forming a platelet plug. These platelets have thrombin receptors on their surfaces that bind serum thrombin molecules, which in turn convert soluble fibrinogen in the serum into fibrin at the wound site. Fibrin forms long strands of tough insoluble protein that are bound to the platelets. Factor XIII completes the cross-linking of fibrin so that it hardens and contracts. The cross-linked fibrin forms a mesh atop the platelet plug that completes the clot. Fibrin was discovered by Marcello Malpighi in 1666. Role in disease Excessive generation of fibrin due to activation of the coagulation cascade leads to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Factor XIII
Factor XIII or fibrin stabilizing factor is a zymogen found in blood of humans and some other animals. It is activated by thrombin to factor XIIIa. Factor XIIIa is an enzyme of the blood coagulation system that crosslinks fibrin. Deficiency of XIII worsens clot stability and increases bleeding tendency. Human XIII is a heterotetramer. It consists of 2 enzymatic A peptides and 2 non-enzymatic B peptides. XIIIa is a dimer of activated A peptides. Function Within blood, thrombins cleave fibrinogens to fibrins during coagulation and a fibrin-based blood clot forms. Factor XIII is a transglutaminase that circulates in human blood as a heterotetramer of two A and two B subunits. Factor XIII binds to the clot via their B units. In the presence of fibrins, thrombin efficiently cleaves the R37– G38 peptide bond of each A unit within a XIII tetramer. A units release their N-terminal activation peptides. Both of the non- covalently bound B units are now able to dissociate from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Factor XI
Factor XI or plasma thromboplastin antecedent is the zymogen form of factor XIa, one of the enzymes of the coagulation cascade. Like many other coagulation factors, it is a serine protease. In humans, Factor XI is encoded by the ''F11'' gene. Function Factor XI (FXI) is produced by the liver and circulates as a homo-dimer in its inactive form. The plasma half-life of FXI is approximately 52 hours. The zymogen factor is activated into ''factor XIa'' by factor XIIa (FXIIa), thrombin, and FXIa itself; due to its activation by FXIIa, FXI is a member of the "contact pathway" (which includes HMWK, prekallikrein, factor XII, factor XI, and factor IX). Factor XIa activates factor IX by selectively cleaving arg- ala and arg-val peptide bonds. Factor IXa, in turn, forms a complex with Factor VIIIa (FIXa-FVIIIa) and activates factor X. Physiological inhibitors of factor XIa include protein Z-dependent protease inhibitor (ZPI, a member of the serine protease inhibitor/se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clotting Factors
Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin. Coagulation begins almost instantly after an injury to the endothelium lining a blood vessel. Exposure of blood to the subendothelial space initiates two processes: changes in platelets, and the exposure of subendothelial tissue factor to plasma factor VII, which ultimately leads to cross-linked fibrin formation. Platelets immediately form a plug at the site of injury; this is called ''primary hemostasis. Secondary hemostasis'' occurs simultaneously: additional coagulation (clotting) factors beyond factor VII ( listed below) respond in a cascade to form fibrin strands, which strengthen the platelet plug. Disorders of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tubulin
Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. α- and β-tubulins polymerize into microtubules, a major component of the eukaryotic cytoskeleton. Microtubules function in many essential cellular processes, including mitosis. Tubulin-binding drugs kill cancerous cells by inhibiting microtubule dynamics, which are required for DNA segregation and therefore cell division. In eukaryotes, there are six members of the tubulin superfamily, although not all are present in all species.Turk E, Wills AA, Kwon T, Sedzinski J, Wallingford JB, Stearns "Zeta-Tubulin Is a Member of a Conserved Tubulin Module and Is a Component of the Centriolar Basal Foot in Multiciliated Cells"Current Biology (2015) 25:2177-2183. Both α and β tubulins have a mass of around 50 kDa and are thus in a similar range compared to actin (with a mass of ~42 kDa). In contrast, tubulin polymers (microtubules) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
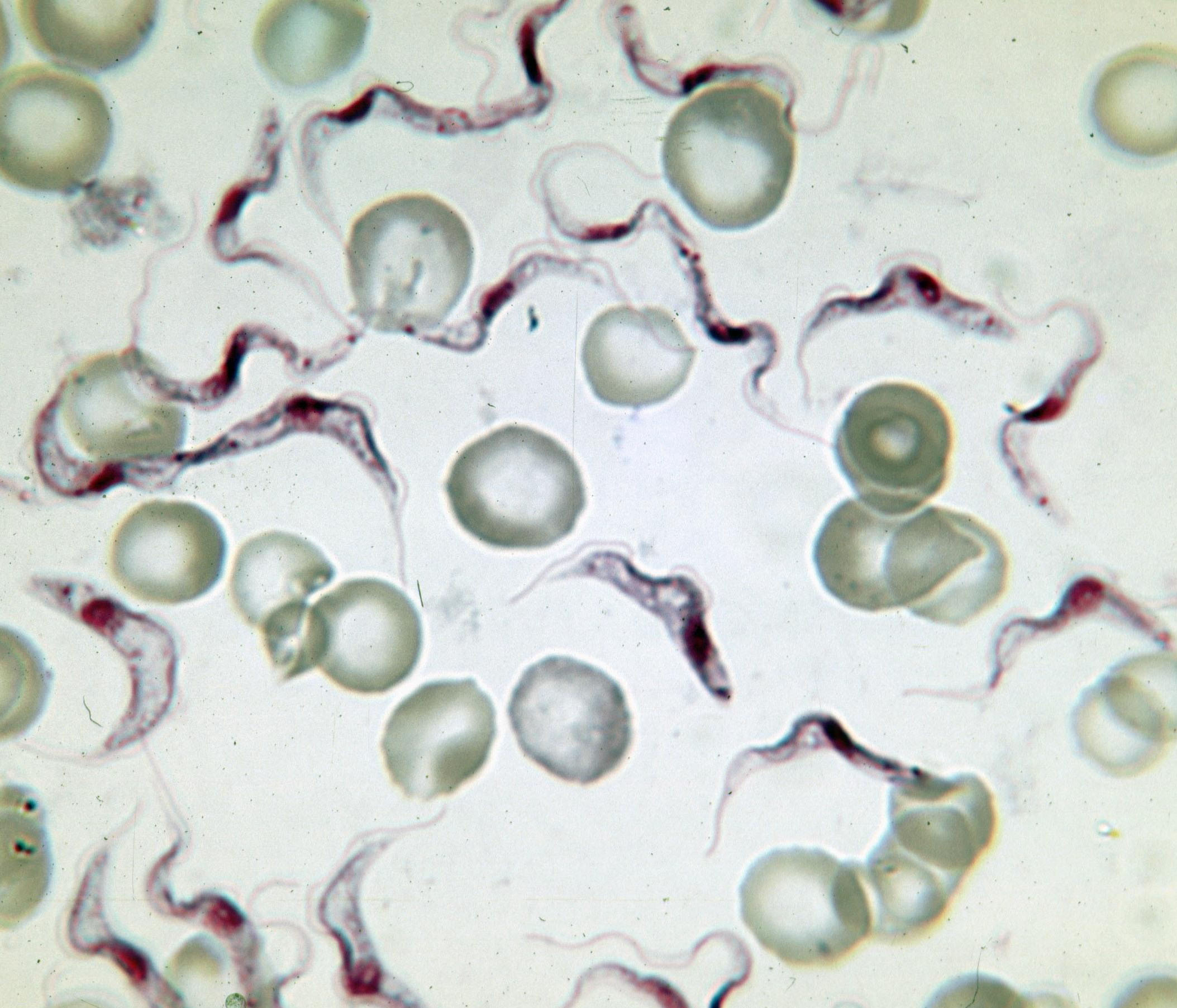
Variable Surface Glycoprotein
Variant surface glycoprotein (VSG) is a ~60kDa protein which densely packs the cell surface of protozoan parasites belonging to the genus ''Trypanosoma''. This genus is notable for their cell surface proteins. They were first isolated from ''Trypanosoma brucei'' in 1975 by George Cross. VSG allows the trypanosomatid parasites to evade the mammalian host's immune system by extensive antigenic variation. They form a 12–15 nm surface coat. VSG dimers, ~90% of all cell surface protein. It also makes up ~10% of total cell protein. For this reason, these proteins are highly immunogenic and an immune response raised against a specific VSG coat will rapidly kill trypanosomes expressing this variant. However, with each cell division there is a possibility that the progeny will switch expression to change the VSG that is being expressed. VSG has no prescribed biochemical activity. The parasite has a large cellular repertoire of antigenically distinct VSGs (~1500/2000 complete and partia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
14-3-3 Protein
14-3-3 proteins are a family of conserved regulatory molecules that are expressed in all eukaryotic cells. 14-3-3 proteins have the ability to bind a multitude of functionally diverse signaling proteins, including kinases, phosphatases, and transmembrane receptors. More than 200 signaling proteins have been reported as 14-3-3 ligands. Elevated amounts of 14-3-3 proteins in cerebrospinal fluid may be a sign of Creutzfeldt–Jakob disease. Properties Seven genes encode seven distinct 14-3-3 proteins in most mammals (See ''Human genes'' below) and 13-15 genes in many higher plants, though typically in fungi they are present only in pairs. Protists have at least one. Eukaryotes can tolerate the loss of a single 14-3-3 gene if multiple genes are expressed, but deletion of all 14-3-3s (as experimentally determined in yeast) results in death. 14-3-3 proteins are structurally similar to the Tetratrico Peptide Repeat (TPR) superfamily, which generally have 9 or 10 alpha helices, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |