|
öý-Hydroxypropionic Acid
3-Hydroxypropionic acid is a carboxylic acid, specifically a beta hydroxy acid. It is an acidic viscous liquid with a pKa of 4.5. It is very soluble in water, soluble in ethanol and diethyl ether. Upon distillation, it dehydrates to form acrylic acid, and is occasionally called hydracrylic acid 3-Hydroxypropionic acid is used in the industrial production of various chemicals such as acrylates. Synthesis 3-Hydroxypropionic acid can be obtained by base-induced hydration of acrylic acid followed by reacidification. Another synthesis involves cyanation of ethylene chlorohydrin followed by hydrolysis of the resulting nitrile. Hydrolysis of propiolactone is yet another route. Potential applications The polyester poly(3-hydroxypropionic acid) is a biodegradable polymer. The method combines the high-molecular weight and control aspects of ring-opening polymerization with the commercial availability of the beta hydroxy acid, 3-hydroxypropionic acid which is abbreviated as 3-HP. Since ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a Volatility (chemistry), volatile, Combustibility and flammability, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of Carbohydrate, sugars by yeasts or via Petrochemistry, petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the Chemical synthesis, synthesis of organic compounds, and as a Alcohol fuel, fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactic Acid
Lactic acid is an organic acid. It has a molecular formula . It is white in the solid state and it is miscible with water. When in the dissolved state, it forms a colorless solution. Production includes both artificial synthesis as well as natural sources. Lactic acid is an alpha-hydroxy acid (AHA) due to the presence of a hydroxyl group adjacent to the carboxyl group. It is used as a synthetic intermediate in many organic synthesis industries and in various biochemical industries. The conjugate base of lactic acid is called lactate (or the lactate anion). The name of the derived acyl group is lactoyl. In solution, it can ionize by loss of a proton to produce the lactate ion . Compared to acetic acid, its p''K'' is 1 unit less, meaning lactic acid is ten times more acidic than acetic acid. This higher acidity is the consequence of the intramolecular hydrogen bonding between the öÝ-hydroxyl and the carboxylate group. Lactic acid is chiral, consisting of two enantiomers. One ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microbes
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, çüö°öÝö§ö¿üö¥üü, ''organismû°s'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in older texts. The informal synonym ''microbe'' () comes from ö¥ö¿ö¤üüü, mikrû°s, "small" and öýö₤ö¢ü, bûÙos, "life". is an organism of microscopic size, which may exist in its single-celled form or as a colony of cells. The possible existence of unseen microbial life was suspected from ancient times, such as in Jain scriptures from sixth century BC India. The scientific study of microorganisms began with their observation under the microscope in the 1670s by Anton van Leeuwenhoek. In the 1850s, Louis Pasteur found that microorganisms caused food spoilage, debunking the theory of spontaneous generation. In the 1880s, Robert Koch discovered that microorganisms caused the diseases tuberculosis, cholera, diphtheria, and anthrax. Because micr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
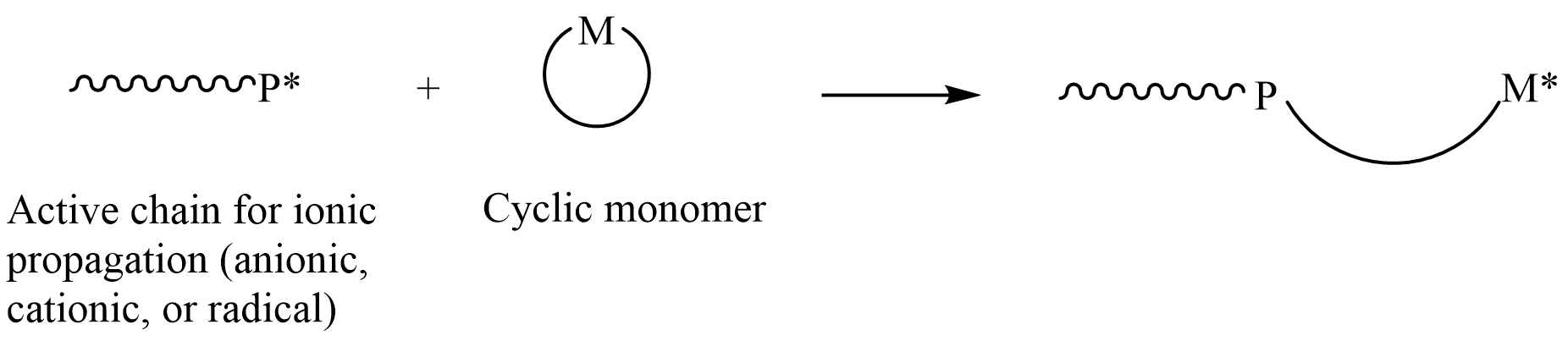
Ring-opening Polymerization
In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization, in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer (see figure). The reactive center can be radical, anionic or cationic. Some cyclic monomers such as norbornene or cyclooctadiene can be polymerized to high molecular weight polymers by using metal catalysts. ROP is a versatile method for the synthesis of biopolymers. Ring-opening of cyclic monomers is often driven by the relief of bond-angle strain. Thus, as is the case for other types of polymerization, the enthalpy change in ring-opening is negative. Monomers Cyclic monomers that are amenable to ROP include epoxides, cyclic trisiloxanes, some lactones, lactides, cyclic carbonates, and amino acid N-carboxyanhydrides. Many strained cycloalkenes, e.g norbornene, are suitable monomers via ring-opening metathesis polymerization. History Ring-opening polymerization has been used since the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biodegradable Polymer
Biodegradable polymers are a special class of polymer that breaks down after its intended purpose by bacterial decomposition process to result in natural byproducts such as gases ( CO2, N2), water, biomass, and inorganic salts. These polymers are found both naturally and synthetically made, and largely consist of ester, amide, and ether functional groups. Their properties and breakdown mechanism are determined by their exact structure. These polymers are often synthesized by condensation reactions, ring opening polymerization, and metal catalysts. There are vast examples and applications of biodegradable polymers. Bio-based packaging materials have been introduced as a green alternative in the past decades, among which, edible films have gained more attention due to their environmentally-friendly characteristics, vast variety and availability, non-toxicity, and low cost. History Biodegradable polymers have a long history, and since many are natural products, the precise timel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyester
Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing. Polyester fibers are sometimes spun together with natural fibers to produce a cloth with blended properties. Cotton-polyester blends can be strong, wrinkle- and tear-resistant, and reduce shrinking. Synthetic fibers using polyester have high water, wind and environmental resistance compared to plant-derived fibers. They are less Fireproofing, fire-resistant and can melt when ignited. Liquid crystalline polyesters are among the first industrially used liquid crystal polymers. They are use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propiolactone
Propiolactone may refer to either of two isomeric chemical compounds: * Alpha-Propiolactone (öÝ-Propiolactone) * Beta-Propiolactone öý-Propiolactone is an organic compound of the lactone family, with a four-membered ring. It is a colorless liquid with a slightly sweet odor, highly soluble in water and miscible with ethanol, acetone, diethyl ether and chloroform.''Merck Index ... (öý-Propiolactone) {{Short pages monitor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acrylate
Acrylates (IUPAC: prop-2-enoates) are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion C H2=CHC OOã. Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain vinyl groups. These compounds are of interest because they are bifunctional: the vinyl group is susceptible to polymerization and the carboxylate group carries myriad functionalities. Modified acrylates are also numerous, some examples being methacrylates (CH2=C(CH3)CO2R) and cyanoacrylates (CH2=C(CN)CO2R). Acrylate can also refer to polyacrylates prepared through the polymerization of the vinyl groups of acrylate monomers. File:Acrylate-anion.svg, The acrylate anion File:Trimethylolpropane triacrylate.svg, Trimethylolpropane triacrylate (TMPTA), a trifunctional acrylate ester File:Methylacrylat.svg, Methyl acrylate, an acrylic ester File:Hexandioldiacrylat.svg, Hexandiol diacrylate, a bifunctional acrylate File:Methyl-meth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acrylic Acid
Acrylic acid (IUPAC: propenoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols, ethers, and chloroform. More than a million tons are produced annually. History The word "acrylic" was coined in 1843, for a chemical derivative of acrolein, an acrid-smelling oil derived from glycerol. Production Acrylic acid is produced by oxidation of propylene, which is a byproduct of the production of ethylene and gasoline: : 2 CH2=CHCH3 + 3 O2 ã 2 CH2=CHCO2H + 2 H2O Historical methods Because acrylic acid and its esters have long been valued commercially, many other methods have been developed. Most have been abandoned for economic or environmental reasons. An early method was the hydrocarboxylation of acetylene (" Reppe chemistry"): : This method re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydration Reaction
In chemistry, a dehydration reaction is a chemical reaction that involves the loss of water from the reacting molecule or ion. Dehydration reactions are common processes, the reverse of a hydration reaction. Dehydration reactions in organic chemistry Esterification The classic example of a dehydration reaction is the Fischer esterification, which involves treating a carboxylic acid with an alcohol to give an ester :RCO2H + RãýOH RCO2Rãý + H2O Often such reactions require the presence of a dehydrating agent, i.e. a substance that reacts with water. Etherification Two monosaccharides, such as glucose and fructose, can be joined together (to form saccharose) using dehydration synthesis. The new molecule, consisting of two monosaccharides, is called a disaccharide. Nitrile formation Nitriles are often prepared by dehydration of primary amides. :RC(O)NH2 ã RCN + H2O Ketene formation Ketene is produced by heating acetic acid and trapping the product: :CH3CO2H ã CH2=C= ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general anesthetic, until non-flammable drugs were developed, such as halothane. It has been used as a recreational drug to cause intoxication. Production Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises. Vapor-phase dehydration of ethanol over some alumina catalysts can give diethyl ether yields of up to 95%. Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis. Ethanol is mixed with a stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |