|
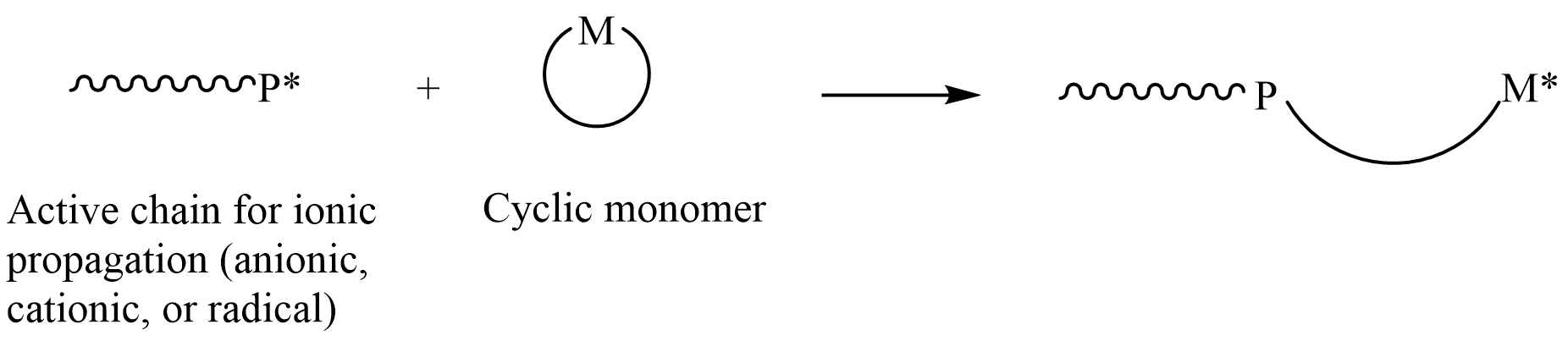
Ring-opening Polymerization
In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization, in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer (see figure). The reactive center can be radical, anionic or cationic. Some cyclic monomers such as norbornene or cyclooctadiene can be polymerized to high molecular weight polymers by using metal catalysts. ROP is a versatile method for the synthesis of biopolymers. Ring-opening of cyclic monomers is often driven by the relief of bond-angle strain. Thus, as is the case for other types of polymerization, the enthalpy change in ring-opening is negative. Monomers Cyclic monomers that are amenable to ROP include epoxides, cyclic trisiloxanes, some lactones, lactides, cyclic carbonates, and amino acid N-carboxyanhydrides. Many strained cycloalkenes, e.g norbornene, are suitable monomers via ring-opening metathesis polymerization. History Ring-opening polymerization has been used since the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Union Of Pure And Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. This administrative office is headed by IUPAC's executive director, currently Lynn Soby. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Mass
The molecular mass (''m'') is the mass of a given molecule: it is measured in daltons (Da or u). Different molecules of the same compound may have different molecular masses because they contain different isotopes of an element. The related quantity relative molecular mass, as defined by IUPAC, is the ratio of the mass of a molecule to the unified atomic mass unit (also known as the dalton) and is unitless. The molecular mass and relative molecular mass are distinct from but related to the molar mass. The molar mass is defined as the mass of a given substance divided by the amount of a substance and is expressed in g/mol. That makes the molar mass an average of many particles or molecules, and the molecular mass the mass of one specific particle or molecule. The molar mass is usually the more appropriate figure when dealing with macroscopic (weigh-able) quantities of a substance. The definition of molecular weight is most authoritatively synonymous with relative molecular mass; ho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dextran
Dextran is a complex branched glucan (polysaccharide derived from the condensation of glucose), originally derived from wine. IUPAC defines dextrans as "Branched poly-α-d-glucosides of microbial origin having glycosidic bonds predominantly C-1 → C-6". Dextran chains are of varying lengths (from 3 to 2000 kilodaltons). The polymer main chain consists of α-1,6 glycosidic linkages between glucose monomers, with branches from α-1,3 linkages. This characteristic branching distinguishes a dextran from a dextrin, which is a straight chain glucose polymer tethered by α-1,4 or α-1,6 linkages. Occurrence Dextran was discovered by Louis Pasteur as a microbial product in wine, but mass production was only possible after the development by Allene Jeanes of a process using bacteria. Dental plaque is rich in dextrans. Dextran is a complicating contaminant in the refining of sugar because it elevates the viscosity of sucrose solutions and fouls plumbing. Dextran is now produced fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polysaccharides
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with water (hydrolysis) using amylase enzymes as catalyst, which produces constituent sugars (monosaccharides, or oligosaccharides). They range in structure from linear to highly branched. Examples include storage polysaccharides such as starch, glycogen and galactogen and structural polysaccharides such as cellulose and chitin. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure, these macromolecules can have distinct properties from their monosaccharide building blocks. They may be amorphous or even insoluble in water. When all the monosaccharides in a polysaccharide are the same type, the polysaccharide is called a homopolysaccharide or homoglycan, but when more t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sugars
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double sugars, are molecules made of two bonded monosaccharides; common examples are sucrose (glucose + fructose), lactose (glucose + galactose), and maltose (two molecules of glucose). White sugar is a refined form of sucrose. In the body, compound sugars are hydrolysed into simple sugars. Longer chains of monosaccharides (>2) are not regarded as sugars, and are called oligosaccharides or polysaccharides. Starch is a glucose polymer found in plants, the most abundant source of energy in human food. Some other chemical substances, such as glycerol and sugar alcohols, may have a sweet taste, but are not classified as sugar. Sugars are found in the tissues of most plants. Honey and fruits are abundant natural sources of simple sugars. Sucrose is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polypeptides
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic peptides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ring-opening Metathesis Polymerization
Ring-opening metathesis polymerization (ROMP) is a type of olefin metathesis chain-growth polymerization. The driving force of the reaction is relief of ring strain in cyclic olefins (e.g. norbornene or cyclopentene). A variety of heterogeneous and homogeneous catalysts have been developed. Most large-scale commercial processes rely on the former while some fine chemical syntheses rely on the homogeneous catalysts. Catalysts are based on transition metals such as W, Mo, Re, Ru, and Ti. Heterogeneous catalysis and applications : Ring-opening metathesis polymerization of cycloalkenes has been commercialized since the 1970s. Examples of polymers produced on an industrial level through ROMP catalysis are Vestenamer or trans-polyoctenamer, which is the metathetical polymer of cyclooctene. Norsorex or polynorbornene is another important ROMP product on the market. Telene and Metton are polydicyclopentadiene products produced in a side reaction of the polymerization of norbornene. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid N-carboxyanhydride
Amino acid ''N''-carboxyanhydrides, also called Leuchs' anhydrides, are a family of heterocyclic organic compounds derived from amino acids. They are white, moisture-reactive solids. They have been evaluated for applications the field of biomaterials. Preparation 140px, Glycine N-carboxyanhydride is the parent member of the amino acid N-carboxyanhydrides. NCAs are typically prepared by phosgenation of amino acids: They were first synthesized by Hermann Leuchs by heating an ''N''-ethoxycarbonyl or ''N''-methoxycarbonyl amino acid chloride in a vacuum at 50-70 °C: A moisture-tolerant route to unprotected NCAs employs epoxides as scavengers of hydrogen chloride. This synthesis of NCAs is sometimes called the . The relatively high temperatures necessary for this cyclization results in the decomposition of several NCAs. Of several improvements, one notable procedure involves treating an unprotected amino acid with phosgene or its trimer. Reactions NCAs are prone to hy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Carbonate
In organic chemistry, a carbonate ester (organic carbonate or organocarbonate) is an ester of carbonic acid. This functional group consists of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is and they are related to esters (), ethers () and also to the inorganic carbonates. Monomers of polycarbonate (e.g. Makrolon or Lexan) are linked by carbonate groups. These polycarbonates are used in eyeglass lenses, compact discs, and bulletproof glass. Small carbonate esters like dimethyl carbonate, ethylene carbonate, propylene carbonate are used as solvents, dimethyl carbonate is also a mild methylating agent. Structures Carbonate esters have planar OC(OC)2 cores, which confers rigidity. The unique O=C bond is short (1.173 Å in the depicted example), while the C-O bonds are more ether-like (the bond distances of 1.326 Å for the example depicted). Carbonate esters can be divided into three structural classes: acyclic, cyclic, and polymeric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactide
Lactide is the lactone cyclic ester derived by multiple esterification between two (usually) or more molecules from lactic acid (2-hydroxypropionic acid) or other hydroxy carboxylic acid. They are designated as dilactides, trilactides, etc., according to the number of hydroxy acid residues. The dilactide derived from lactic acid has the formula (OCHMeCO2)2. All lactides are colorless or white solids. This lactide has attracted interest because it is derived from abundant renewable resources and is the precursor to a biodegradable a plastic. Stereoisomers The dilactide derived from lactic acid can exist in three different stereoisomeric forms. This complexity arises because lactic acid is chiral. These enantiomers do not racemize readily. All three stereoisomers undergo epimerisation in the presence of organic and inorganic bases in solution. Polymerization Lactide can be polymerized to polylactic acid (polylactide). Depending on the catalyst, syndiotactic Tacticity (fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile. Nomenclature A compound containing the epoxide functional group can be called an epoxy, epoxide, oxirane, and ethoxyline. Simple epoxides are often referred to as oxides. Thus, the epoxide of ethylene (C2H4) is ethylene oxide (C2H4O). Many compounds have trivial names; for instance, ethylene oxide is called "oxirane". Some names emphasize the presence of the epoxide functional group, as in the compound ''1,2-epoxyheptane'', which can also be called ''1,2-heptene oxide''. A polymer formed from epoxide precursors is called an ''epoxy'', but such materials do not contain epoxide groups (or contain only a few residual epoxy grou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant pressure, which is conveniently provided by the large ambient atmosphere. The pressure–volume term expresses the work required to establish the system's physical dimensions, i.e. to make room for it by displacing its surroundings. The pressure-volume term is very small for solids and liquids at common conditions, and fairly small for gases. Therefore, enthalpy is a stand-in for energy in chemical systems; bond, lattice, solvation and other "energies" in chemistry are actually enthalpy differences. As a state function, enthalpy depends only on the final configuration of internal energy, pressure, and volume, not on the path taken to achieve it. In the International System of Units (SI), the unit of measurement for enthalpy is the joule. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |