Ring-opening Polymerization on:
[Wikipedia]
[Google]
[Amazon]
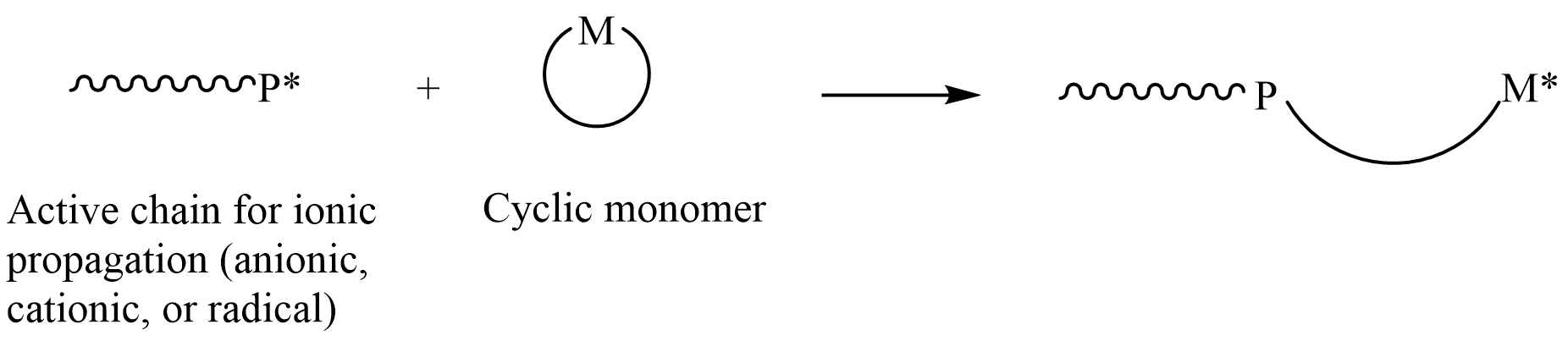 In
In
 The monomers can be activated by Bronsted acids,
The monomers can be activated by Bronsted acids,
 Commercially relevant unsaturated polymers synthesized by ROMP include Norsorex (
Commercially relevant unsaturated polymers synthesized by ROMP include Norsorex (
Olefin Metathesis Polymerization
 In
In polymer chemistry
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures of chemicals, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are ...
, ring-opening polymerization (ROP) is a form of chain-growth polymerization, in which the terminus of a polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
chain attacks cyclic monomers to form a longer polymer (see figure). The reactive center can be radical, anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
ic or cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
ic. Some cyclic monomers such as norbornene or cyclooctadiene can be polymerized
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many for ...
to high molecular weight
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
polymers by using metal catalysts
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
. ROP is a versatile method for the synthesis of biopolymers.
Ring-opening of cyclic monomers is often driven by the relief of bond-angle strain. Thus, as is the case for other types of polymerization, the enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
change in ring-opening is negative.
Monomers
Cyclic monomers that are amenable to ROP includeepoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale ...
s, cyclic trisiloxanes, some lactones, lactide
Lactide is the lactone cyclic ester derived by multiple esterification between two (usually) or more molecules from lactic acid (2-hydroxypropionic acid) or other hydroxy carboxylic acid. They are designated as dilactides, trilactides, etc., acco ...
s, cyclic carbonate
In organic chemistry, a carbonate ester (organic carbonate or organocarbonate) is an ester of carbonic acid. This functional group consists of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is and they ...
s, and amino acid N-carboxyanhydride
Amino acid ''N''-carboxyanhydrides, also called Leuchs' anhydrides, are a family of heterocyclic organic compounds derived from amino acids. They are white, moisture-reactive solids. They have been evaluated for applications the field of biomate ...
s. Many strained cycloalkenes, e.g norbornene, are suitable monomers via ring-opening metathesis polymerization.
History
Ring-opening polymerization has been used since the beginning of the 1900s to producepolymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
s. Synthesis of polypeptides
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A p ...
which has the oldest history of ROP, dates back to the work in 1906 by Leuchs. Subsequently, the ROP of anhydro sugars
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double ...
provided polysaccharides
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with w ...
, including synthetic dextran
Dextran is a complex branched glucan ( polysaccharide derived from the condensation of glucose), originally derived from wine. IUPAC defines dextrans as "Branched poly-α-d-glucosides of microbial origin having glycosidic bonds predominantly C-1 ...
, xanthan gum
Xanthan gum () is a polysaccharide with many industrial uses, including as a common food additive. It is an effective thickening agent, emulsifier, and stabilizer that prevents ingredients from separating. It can be produced from simple sugars ...
, welan gum, gellan gum
Gellan gum is a water-soluble anionic polysaccharide produced by the bacterium '' Sphingomonas elodea'' (formerly ''Pseudomonas elodea'' based on the taxonomic classification at the time of its discovery). The gellan-producing bacterium was disco ...
, diutan gum, and pullulan
Pullulan is a polysaccharide polymer consisting of maltotriose units, also known as α-1,4- ;α-1,6- glucan'. Three glucose units in maltotriose are connected by an α-1,4 glycosidic bond, whereas consecutive maltotriose units are connected to e ...
. Mechanisms and thermodynamics of ring-opening polymerization were established in the 1950s. The first high-molecular weight polymers (Mn up to 105) with a repeating unit
In polymer chemistry, a repeat unit or repeating unit (or mer) is a part of a polymer whose repetition would produce the complete polymer chain (except for the end-groups) by linking the repeat units together successively along the chain, like th ...
were prepared by ROP as early as in 1976.
An industrial application is the production of nylon-6
Nylon 6 or polycaprolactam is a polymer, in particular semicrystalline polyamide. Unlike most other nylons, nylon 6 is not a condensation polymer, but instead is formed by ring-opening polymerization; this makes it a special case in the comp ...
.
Mechanisms
Ring-opening polymerization can proceed via radical, anionic, or cationic polymerization as described below. Additionally, radical ROP is useful in producing polymers withfunctional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
s incorporated in the backbone chain that cannot otherwise be synthesized via conventional chain-growth polymerization of vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl m ...
monomers. For instance, radical ROP can produce polymers with ethers
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be ...
, esters
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are ...
, amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it i ...
s, and carbonates
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate g ...
as functional groups along the main chain.
Anionic ring-opening polymerization (AROP)
Anionic ring-opening polymerizations (AROP) involve nucleophilic reagents as initiators. Monomers with a three-member ring structure - such asepoxides
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale f ...
, aziridines
220 px, chemotherapeutic agent by virtue of its antitumour activity. Aziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine (-NR-) and two methylene bridges (--). The parent compou ...
, and episulfides - undergo anionic ROP.
A typical example of anionic ROP is that of ε-caprolactone, initiated by an alkoxide.
Cationic ring-opening polymerization
Cationic initiators and intermediates characterize cationic ring-opening polymerization (CROP). Examples of cyclic monomers that polymerize through this mechanism includelactone
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring.
Lactones are formed by intramolecular esterification of the co ...
s, lactam
A lactam is a cyclic amide, formally derived from an amino alkanoic acid. The term is a portmanteau of the words ''lactone'' + ''amide''.
Nomenclature
Greek prefixes in alphabetical order indicate ring size:
* α-Lactam (3-atom rings)
* β-Lacta ...
s, amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
s, and ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be c ...
s. CROP proceeds through an SN1 or SN2 propagation, chain-growth process. The mechanism is affected by the stability of the resulting cationic
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
species. For example, if the atom bearing the positive charge is stabilized by electron-donating groups, polymerization will proceed by the SN1 mechanism. The cationic species is a heteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is usually used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecula ...
and the chain grows by the addition of cyclic monomers thereby opening the ring system.
carbenium ion
A carbenium ion is a positive ion with the structure RR′R″C+, that is, a chemical species with a trivalent carbon that bears a +1 formal charge.
In older literature the name carbonium ion was used for this class, but now it refers exclusivel ...
s, onium ions, and metal cations.
CROP can be a living polymerization
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer ...
and can be terminated by nucleophilic reagents such as phenoxy anions, phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
s, or polyanions
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are th ...
. When the amount of monomers becomes depleted, termination can occur intra or intermolecularly. The active end can "backbite" the chain, forming a macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
...
. Alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalk ...
chain transfer is also possible, where the active end is quenched by transferring an alkyl chain to another polymer.
Ring-opening metathesis polymerization
Ring-opening metathesis polymerization (ROMP) produces unsaturated polymers fromcycloalkene
A cycloalkene or cycloolefin is a type of alkene hydrocarbon which contains a closed ring of carbon atoms and either one or more double bonds, but has no aromatic character. Some cycloalkenes, such as cyclobutene and cyclopentene, can be used as m ...
s or bicycloalkenes. It requires organometallic catalysts.
The mechanism for ROMP follows similar pathways as olefin metathesis
Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often create ...
. The initiation process involves the coordination of the cycloalkene monomer to the metal alkylidene complex, followed by a +2type cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". T ...
to form the metallacyclobutane intermediate that cycloreverts to form a new alkylidene species.
 Commercially relevant unsaturated polymers synthesized by ROMP include Norsorex (
Commercially relevant unsaturated polymers synthesized by ROMP include Norsorex (polynorbornene
Norbornene or norbornylene or norcamphene is a highly strained bridged cyclic hydrocarbon. It is a white solid with a pungent sour odor. The molecule consists of a cyclohexene ring with a methylene bridge between carbons 1 and 4. The molecule carr ...
), Vestenamer (polycyclooctene), and Metton (polycyclopentadiene).
Thermodynamics
The formal thermodynamic criterion of a given monomer polymerizability is related to a sign of thefree enthalpy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and pre ...
(Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and pr ...
) of polymerization:
:
where x and y indicate monomer and polymer states, respectively (x and/or y = l (liquid), g (gaseous
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), ...
), c (amorphous solid
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal.
Etymology
The term comes from the Greek ''a'' ("wit ...
), c’ (crystalline solid
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
), s (solution
Solution may refer to:
* Solution (chemistry), a mixture where one substance is dissolved in another
* Solution (equation), in mathematics
** Numerical solution, in numerical analysis, approximate solutions within specified error bounds
* Soluti ...
)), ΔHp(xy) and ΔSp(xy) are the corresponding enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
(SI unit: joule per kelvin) and entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
(SI unit: joule) of polymerization, and T is the absolute temperature (SI unit: kelvin).
The free enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
of polymerization (ΔGp) may be expressed as a sum of standard enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
of polymerization (ΔGp°) and a term related to instantaneous monomer molecules and growing macromolecules
A macromolecule is a very large molecule important to biophysical processes, such as a protein or nucleic acid. It is composed of thousands of covalently bonded atoms. Many macromolecules are polymers of smaller molecules called monomers. The ...
concentrations:
:
where R is the gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per ...
, M is the monomer, (m)i is the monomer in an initial state, and m* is the active monomer.
Following Flory–Huggins solution theory
Flory–Huggins solution theory is a lattice model of the thermodynamics of polymer solutions which takes account of the great dissimilarity in molecular sizes in adapting the usual expression for the entropy of mixing. The result is an equation ...
that the reactivity of an active center, located at a macromolecule of a sufficiently long macromolecular chain, does not depend on its degree of polymerization
The degree of polymerization, or DP, is the number of monomeric units in a macromolecule or polymer or oligomer molecule.
For a homopolymer, there is only one type of monomeric unit and the ''number-average'' degree of polymerization is given b ...
(DPi), and taking in to account that ΔGp° = ΔHp° - TΔSp° (where ΔHp° and ΔSp° indicate a standard polymerization enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
and entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
, respectively), we obtain:
:
At equilibrium (ΔGp = 0), when polymerization is complete the monomer concentration ( sub>eq) assumes a value determined by standard polymerization parameters (ΔHp° and ΔSp°) and polymerization temperature:
:
:
:
Polymerization is possible only when sub>0 > sub>eq. Eventually, at or above the so-called ceiling temperature Ceiling temperature (T_c) is a measure of the tendency of a polymer to revert to its constituent monomers. When a polymer is at its ceiling temperature, the rate of polymerization and depolymerization of the polymer are equal. Generally, the cei ...
(Tc), at which sub>eq = sub>0, formation of the high polymer does not occur.
:
:
For example, tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is ma ...
(THF) cannot be polymerized above Tc = 84 °C, nor cyclo-octasulfur (S8) below Tf = 159 °C. However, for many monomers, Tc and Tf, for polymerization in the bulk, are well above or below the operable polymerization temperatures, respectively.
The polymerization of a majority of monomers is accompanied by an entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
decrease, due mostly to the loss in the translational degrees of freedom. In this situation, polymerization is thermodynamically allowed only when the enthalpic contribution into ΔGp prevails (thus, when ΔHp° < 0 and ΔSp° < 0, the inequality , ΔHp, > -TΔSp is required). Therefore, the higher the ring strain, the lower the resulting monomer concentration at equilibrium.
See also
*Ring opening metathesis polymerization
Ring-opening metathesis polymerization (ROMP) is a type of olefin metathesis chain-growth polymerization. The driving force of the reaction is relief of ring strain in cyclic olefins (e.g. norbornene or cyclopentene). A variety of heterogeneo ...
Olefin Metathesis Polymerization
Additional reading
* * * * *References