|
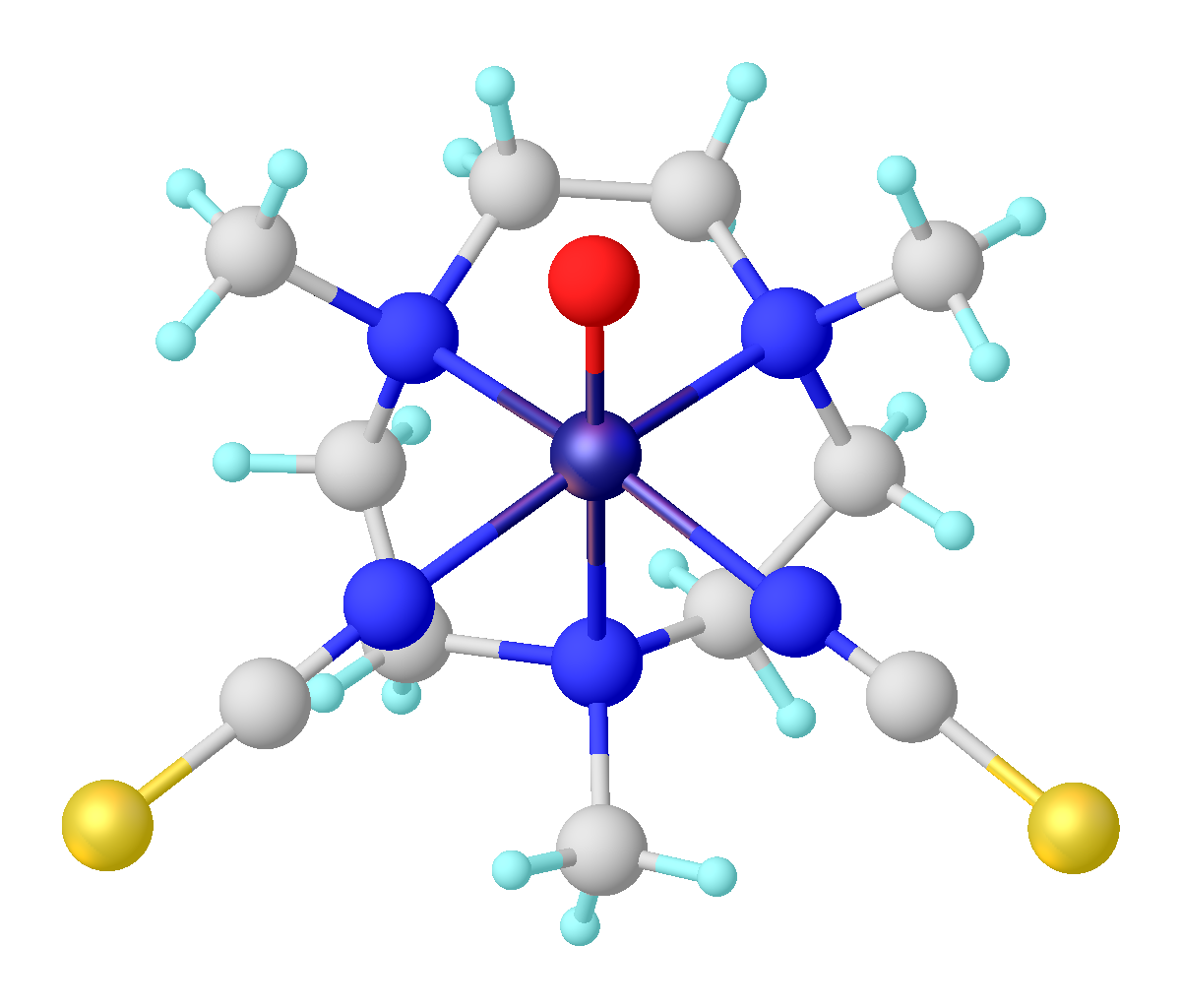
Vanadyl Ion
The vanadyl or oxovanadium(IV) cation, VO2+, is a functional group that is common in the coordination chemistry of vanadium. Complexes containing this functional group are characteristically blue and paramagnetic. A triple bond is proposed to exist between the V4+ and O2− centers. The description of the bonding in the vanadyl ion was central to the development of modern ligand-field theory. Natural occurrence Minerals Cavansite and pentagonite are vanadyl-containing minerals. Water VO2+, often in an ionic pairing with sodium (NaH2VO4), is the second most abundant transition metal in seawater, with its concentration only being exceeded by molybdenum. In the ocean the average concentration is 30 nM. Some mineral water springs also contain the ion in high concentrations. For example, springs near Mount Fuji often contain as much as 54 μg per liter. Vanadyl containing compounds Oxovanadium(IV) * vanadyl acetylacetonate, VO(acac)2 * vanadyl sulfate pentah ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pervanadyl
Pervanadyl () is a pale yellow oxycation of vanadium(V). It is the predominant vanadium(V) species in acidic solutions with pH between 0 and 2, and its salts are formed by protonation of vanadium(V) oxide in such solutions: : ('' K'' = ) The ion can form a complex with a single aminopolycarboxylate ligand, or with tridentate Schiff base ligands. The / redox couple is used at the cathode of the vanadium redox battery. The standard reduction potential of this couple is +1.00 V. See also * Vanadate In chemistry, a vanadate is an anionic coordination complex of vanadium. Often vanadate refers to oxoanions of vanadium, most of which exist in its highest oxidation state of +5. The complexes and are referred to as hexacyanovanadate(III) and no ..., vanadium(V) oxyanions References {{reflist Vanadyl compounds Vanadium(V) compounds Oxycations ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liter
The litre (international spelling) or liter (American English spelling) (SI symbols L and l, other symbol used: ℓ) is a metric unit of volume. It is equal to 1 cubic decimetre (dm3), 1000 cubic centimetres (cm3) or 0.001 cubic metre (m3). A cubic decimetre (or litre) occupies a volume of (see figure) and is thus equal to one-thousandth of a cubic metre. The original French metric system used the litre as a base unit. The word ''litre'' is derived from an older French unit, the '' litron'', whose name came from Byzantine Greek—where it was a unit of weight, not volume—via Late Medieval Latin, and which equalled approximately 0.831 litres. The litre was also used in several subsequent versions of the metric system and is accepted for use with the SI,Bureau International des Poids et M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanyl
In inorganic chemistry, titanyl refers to the functional group TiIVO, sometimes written TiO2+. The term titanyl is used loosely to describe many titanium(IV) oxide compounds and complexes. For example, titanyl sulfate and potassium titanyl phosphate contain TiIVO centers with the connectivity O-Ti-O-Ti. In heterogeneous catalysis, titanyl refers to a terminal oxo ligand A transition metal oxo complex is a coordination complex containing an oxo ligand. Formally O2-, an oxo ligand can be bound to one or more metal centers, i.e. it can exist as a terminal or (most commonly) as bridging ligands (Fig. 1). Oxo ligan ... on a surface titanium(IV) center.{{cite journal, title=The Role of Synchrotron-Based Studies in the Elucidation and Design of Active Sites in Titanium−Silica Epoxidation Catalysts, authors=John Meurig Thomas, Gopinathan Sankar, journal=Accounts of Chemical Research, year=2001, volume=34, pages=571-581, doi=10.1021/ar010003w There are a few molecular titanyl comple ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthovanadate
In chemistry, a vanadate is an anionic coordination complex of vanadium. Often vanadate refers to oxoanions of vanadium, most of which exist in its highest oxidation state of +5. The complexes and are referred to as hexacyanovanadate(III) and nonachlorodivanadate(III), respectively. A simple vanadate ion is the tetrahedral orthovanadate anion, (which is also called vanadate(V)), which is present in e.g. sodium orthovanadate and in solutions of in strong base ( pH > 13). Conventionally this ion is represented with a single double bond, however this is a resonance form as the ion is a regular tetrahedron with four equivalent oxygen atoms. Additionally a range of polyoxovanadate ions exist which include discrete ions and "infinite" polymeric ions. There are also vanadates, such as rhodium vanadate, , which has a statistical rutile structure where the and ions randomly occupy the positions in the rutile lattice, that do not contain a lattice of cations and balancing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadyl Perchlorate
Vanadyl perchlorate or vanadyl triperchlorate is a golden yellow coloured liquid or crystalline compound of vanadium, oxygen and perchlorate group. The substance consists of molecules covalently bound and is quite volatile. Formation Vanadyl perchlorate can be made by reacting vanadium pentoxide with dichlorine heptoxide at 5 °C. It is purified by distillation under a vacuum and recrystallisation at 21 °C. A solution of vanadium(V) perchlorate can be made by dissolving vanadium pentoxide in perchloric acid. The reaction of vanadium pentoxide and dichlorine hexoxide Dichlorine hexoxide is the chemical compound with the molecular formula , which is correct for its gaseous state. However, in liquid or solid form, this chlorine oxide ionizes into the dark red ionic compound chloryl perchlorate , which may be tho ... could produce VO(ClO4)3: : 2 V2O5 + 12 Cl2O6 → 4 VO(ClO4)3 + 12 ClO2 + 3 O2 Properties It can react with vanadium oxychloride to form another vanady ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadyl Nitrate
Vanadyl nitrate, also called vanadium oxytrinitrate or vanadium oxynitrate is an inorganic compound of vanadium in the +5 oxidation state with nitrate ligands and oxygen. The formula is VO(NO3)3. It is a pale yellow viscous liquid. Production It is made by soaking vanadium pentoxide in liquid dinitrogen pentoxide for durations around two days at room temperature. The yield for this method is about 85%. :V2O5 + 3 N2O5 → 2 VO(NO3)3. Purification can be achieved by vacuum distillation. Mononitratodioxovanadium (VO2NO3) is an intermediate in this synthesis. It is a brick red solid. Vanadyl nitrate can also be made from vanadyl trichloride VOCl3 and dinitrogen pentoxide. Structure VO(NO3)3 has a distorted pentagonal bipyramid shape with idealized Cs (mirror) symmetry. The vanadium oxygen bond (157.2 pm) is typical for vanadyl(V). Two nitrate groups in the pentagonal plane are bidentate (V-O distances range from 199 to 206 pm). The third nitrate spans the pentagonal plane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropyl
In organic chemistry, propyl is a three-carbon alkyl substituent with chemical formula for the linear form. This substituent form is obtained by removing one hydrogen atom attached to the terminal carbon of propane. A propyl substituent is often represented in organic chemistry with the symbol Pr (not to be confused with the element praseodymium). An isomeric form of propyl is obtained by moving the point of attachment from a terminal carbon atom to the central carbon atom, named 1-methylethyl or isopropyl. To maintain four substituents on each carbon atom, one hydrogen atom has to be moved from the middle carbon atom to the carbon atom which served as attachment point in the ''n''-propyl variant, written as . Linear propyl is sometimes termed normal and hence written with a prefix ''n''- (i.e., ''n-''propyl), as the absence of the prefix ''n''- does not indicate which attachment point is chosen, i.e. absence of prefix does not automatically exclude the possibility of it being ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadyl Isopropoxide
Vanadyl isopropoxide is the metal alkoxide with the formula VO(O-iPr)3 (iPr = CH(CH3)2). A yellow volatile liquid, it is a common alkoxide of vanadium. It is used as a reagent and as a precursor to vanadium oxides. The compound is diamagnetic. It is prepared by alcoholysis of vanadyl trichloride: :VOCl3 + 3 HOCH(CH3)2 → VO(OCH(CH3)2)3 + 3 HCl The related cyclopentanoxide VO(O-CH(CH2)4)3 is a dimer, one pair of alkoxide ligands bind weakly trans to the vanadyl The vanadyl or oxovanadium(IV) cation, VO2+, is a functional group that is common in the coordination chemistry of vanadium. Complexes containing this functional group are characteristically blue and paramagnetic. A triple bond is proposed to ex ... oxygens. References {{Vanadium compounds Vanadium(V) compounds Alkoxides Vanadyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2.png)