Titanyl on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 In
In inorganic chemistry
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disci ...
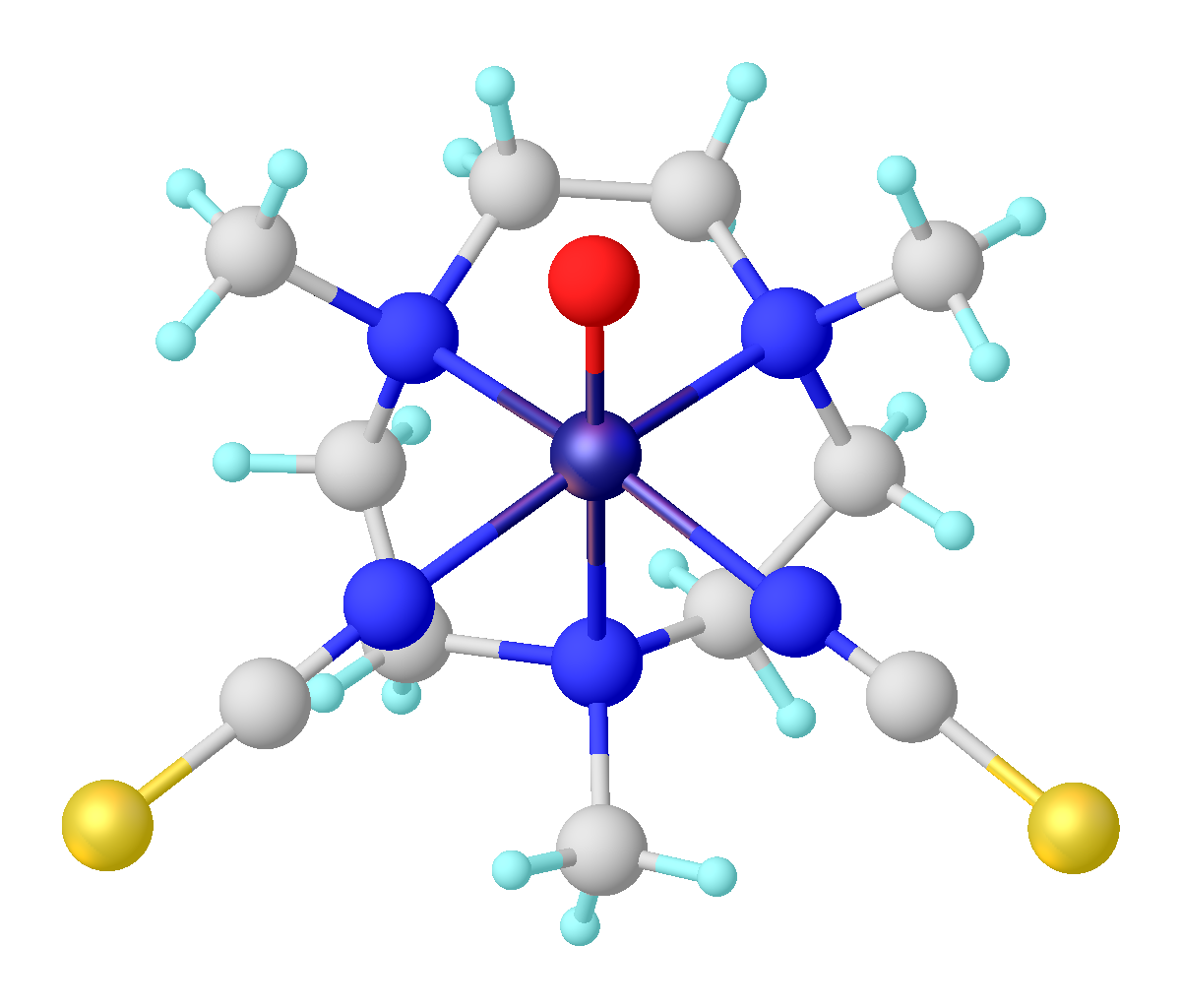
, titanyl refers to the functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
TiIVO, sometimes written TiO2+. The term titanyl is used loosely to describe many titanium(IV) oxide compounds and complexes. For example, titanyl sulfate and potassium titanyl phosphate
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospher ...
contain TiIVO centers with the connectivity O-Ti-O-Ti. In heterogeneous catalysis
In chemistry, heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. Ph ...
, titanyl refers to a terminal oxo ligand
A transition metal oxo complex is a coordination complex containing an oxo ligand. Formally O2-, an oxo ligand can be bound to one or more metal centers, i.e. it can exist as a terminal or (most commonly) as bridging ligands (Fig. 1). Oxo ligan ...
on a surface titanium(IV) center.{{cite journal, title=The Role of Synchrotron-Based Studies in the Elucidation and Design of Active Sites in Titanium−Silica Epoxidation Catalysts, authors=John Meurig Thomas, Gopinathan Sankar, journal=Accounts of Chemical Research, year=2001, volume=34, pages=571-581, doi=10.1021/ar010003w There are a few molecular titanyl complexes where the oxo ligand is terminal, not bridging. In these cases the titanyl group is described as having a triple bond, i.e., Ti≡O.
References