|
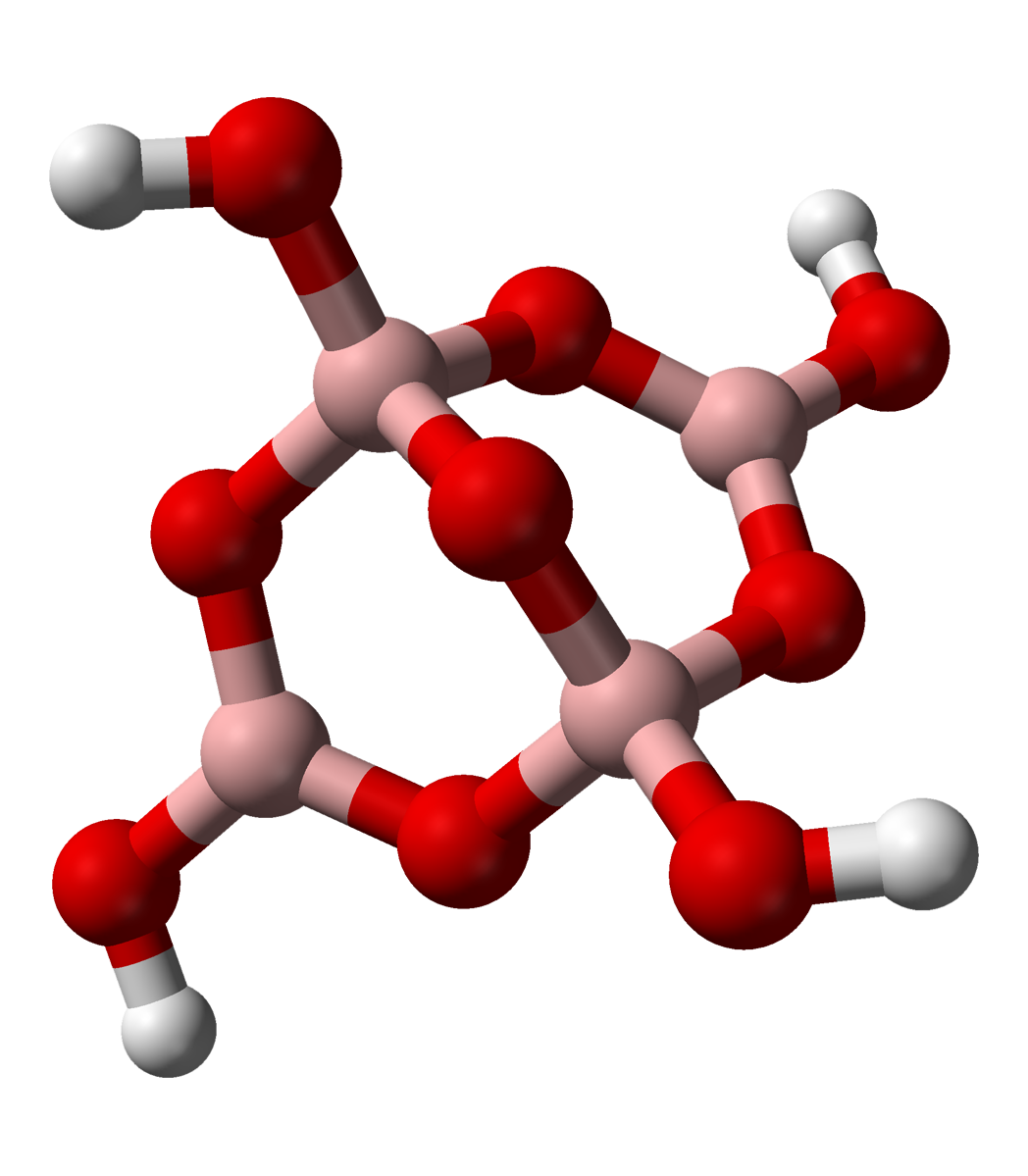
Tetraborate
In chemistry, tetraborate or pyroborate is an anion (negative ion) with formula ; or a salt containing that anion, such as sodium tetraborate, . It is one of the boron oxoacids, that is, a borate. The name is also applied to the hydrated ion as present in borax The ion occurs in boric acid solutions at neutral pH, being formed by condensation of orthoborate and tetrahydroxyborate anions: : 2 B(OH)3 + 2 ⇌ + 5 H2O The tetraborate anion (tetramer) includes two tetrahedral and two trigonal boron atoms symmetrically assembled in a fused bicyclic structure. The two tetrahedral boron atoms are linked together by a common oxygen atom, and each also bears a negative net charge brought by the supplementary OH− groups laterally attached to them. This intricate molecular anion also exhibits three rings: two fused distorted hexagonal (boroxole) rings and one distorted octagonal ring. Each ring is made of a succession of alternate boron and oxygen atoms. Boroxole rings are a very comm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral borax, sodium borate, and the ultra-hard crystals of boron carbide and boron nitride. Boron is synthesized entirely by cosmic ray spallation and supernovae and not by stellar nucleosynthesis, so it is a low-abundance element in the Solar System and in the Crust (geology), Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. It is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known deposits are in Turkey, the largest producer of boron minerals. Elemental b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthoborate
In inorganic chemistry, an orthoborate is a polyatomic anion with formula or a salt containing the anion; such as trisodium orthoborate . It is one of several boron oxoanions, or borates. The name is also used in organic chemistry for the trivalent functional group , or any compound (ester) that contains it, such as triethyl orthoborate . Structure The orthoborate ion is known in the solid state, for example, in calcium orthoborate , where it adopts a nearly trigonal planar structure. It is a structural analogue of the carbonate anion , with which it is isoelectronic. Simple bonding theories point to the trigonal planar structure. In terms of valence bond theory, the bonds are formed by using sp2 hybrid orbitals on boron. Some compounds termed orthoborates do not necessarily contain the trigonal planar ion. For example, gadolinium orthoborate contains the planar ion only a high temperatures; otherwise it contains the polyborate anion . Reactions Solution When orthobora ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salts
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions. The component ions in a salt compound can be either inorganic, such as chloride (Cl−), or organic, such as acetate (). Each ion can be either monatomic, such as fluoride (F−), or polyatomic, such as sulfate (). Types of salt Salts can be classified in a variety of ways. Salts that produce hydroxide ions when dissolved in water are called ''alkali salts'' and salts that produce hydrogen ions when dissolved in water are called ''acid salts''. ''Neutral salts'' are those salts that are neither acidic nor basic. Zwitterions contain an anionic and a cationic centre in the same molecule, but are not considered salts. Examples of zwitterions are amino acids, many metabolites, peptides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Standard Solution
In analytical chemistry, a standard solution is a solution containing a precisely known concentration of an element or a substance. A known mass of solute is dissolved to make a specific volume. It is prepared using a standard substance, such as a primary standard. Standard solutions are used to determine the concentrations of other substances, such as solutions in titration. The concentrations of standard solutions are normally expressed in units of moles per litre (mol/L, often abbreviated to M for molarity), moles per cubic decimetre (mol/dm3), kilomoles per cubic metre (kmol/m3) or in terms related to those used in particular titrations (such as titres). A simple standard is obtained by the dilution of a single element or a substance in a soluble solvent with which it reacts. A primary standard is a reagent that is extremely pure, stable, has no waters of hydration, and has high molecular weight. Some primary standards of titration of acids include sodium carbonate. Uses A known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramer
A tetramer () (''tetra-'', "four" + '' -mer'', "parts") is an oligomer formed from four monomers or subunits. The associated property is called ''tetramery''. An example from inorganic chemistry is titanium methoxide with the empirical formula Ti(OCH3)4, which is tetrameric in solid state and has the molecular formula Ti4(OCH3)16. An example from organic chemistry is kobophenol A, a substance that is formed by combining four molecules of resveratrol. In biochemistry, it similarly refers to a biomolecule formed of four units, that are the same (homotetramer), i.e. as in Concanavalin A or different (heterotetramer), i.e. as in hemoglobin. Hemoglobin has 4 similar sub-units while immunoglobulins have 2 very different sub-units. The different sub-units may have each their own activity, such as binding biotin in avidin tetramers, or have a common biological property, such as the allosteric binding of oxygen in hemoglobin. See also * Cluster chemistry; atomic and molecular clusters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydroxyborate
Tetrahydroxyborate is an inorganic anion with the chemical formula or . It contributes no colour to tetrahydroxyborate salts. It is found in the mineral hexahydroborite, , originally formulated . It is one of the boron oxoanions, and acts as a weak base. The systematic names are tetrahydroxyboranuide (substitutive) and tetrahydroxidoborate(1−) (additive). It can be viewed as the conjugate base of boric acid. Structure Tetrahydroxyborate has a symmetric tetrahedral geometry, isoelectronic with the hypothetical compound orthocarbonic acid . Chemical properties Basicity Tetrahydroxyborate act as a weak Brønsted–Lowry base because it can assimilate a proton (), yielding boric acid with release of water: : + + It can also release a hydroxy anion , thus acting as a classical Arrhenius base: : + (pK = 9.14 to the left) Thus, when boric acid is dissolved in pure (neutral) water, most of it will exist as tetrahydroxyborate ions. With diols In aqueous solution, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borax
Borax is a salt (ionic compound), a hydrated borate of sodium, with chemical formula often written . It is a colorless crystalline solid, that dissolves in water to make a basic solution. It is commonly available in powder or granular form, and has many industrial and household uses, including as a pesticide, as a metal soldering flux, as a component of glass, enamel, and pottery glazes, for tanning of skins and hides, for artificial aging of wood, as a preservative against wood fungus, and as a pharmaceutic alkalizer. In chemical laboratories, it is used as a buffering agent. The compound is often called sodium tetraborate decahydrate, but that name is not consistent with its structure. The anion is not tetraborate but tetrahydroxy tetraborate , so the more correct formula should be . Informally, the product is often called sodium borate decahydrate or just sodium borate. The terms tincal "tinkle" and tincar "tinker" refer to native borax, historically mined from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boric Acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen borate or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolves in water, and occurs in nature as the mineral sassolite. It is a weak acid that yields various borate anions and salts, and can react with alcohols to form borate esters. Boric acid is often used as an antiseptic, insecticide, flame retardant, neutron absorber, or precursor to other boron compounds. The term "boric acid" is also used generically for any oxoacid of boron, such as metaboric acid and tetraboric acid . History Orthoboric acid was first prepared by Wilhelm Homberg (1652–1715) from borax, by the action of mineral acids, and was given the name ("sedative salt of Homberg"). However boric acid and borates have been used since the time of the ancient Greeks for cleaning, preserving food, and other activities. Molecular a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a Chemical reaction, reaction with other Chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borate
A borate is any of several boron oxyanions, negative ions consisting of boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt with such anions, such as sodium metaborate, and disodium tetraborate . The name also refers to certain functional groups in molecules consisting of boron and oxygen, and esters with such groups, such as triethyl orthoborate . Natural occurrence Borate ions occur, alone or with other anions, in many borate and borosilicate minerals such as borax, boracite, ulexite (boronatrocalcite) and colemanite. Borates also occur in seawater, where they make an important contribution to the absorption of low frequency sound in seawater. Borates also occur in plants, including almost all fruits. Anions The main borate anions are: * tetrahydroxyborate , found in sodium tetrahydroxyborate . * orthoborate , found in trisodium orthoborate * perborate , as in sodium perborate * metaborate or , found in sodium metaborate * diborate , f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |