Tetraborate on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 In
In chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, proper ...
, tetraborate or pyroborate is an anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
(negative ion) with formula ; or a salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quant ...
containing that anion, such as sodium tetraborate, . It is one of the boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the '' boron group'' it has t ...
oxoacid
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen atom bonded to oxygen that can dissociate to produce ...
s, that is, a borate
A borate is any of several boron oxyanions, negative ions consisting of boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt with such anions, such as sodium metaborate, and disodium tetraborate . The name also re ...
.
The name is also applied to the hydrated ion as present in borax
Borax is a salt (ionic compound), a hydrated borate of sodium, with chemical formula often written . It is a colorless crystalline solid, that dissolves in water to make a basic solution. It is commonly available in powder or granular form ...
The ion occurs in boric acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen borate or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolve ...
solutions at neutral pH, being formed by condensation of orthoborate and tetrahydroxyborate anions:
: 2 B(OH)3 + 2 Ôçî + 5 H2O
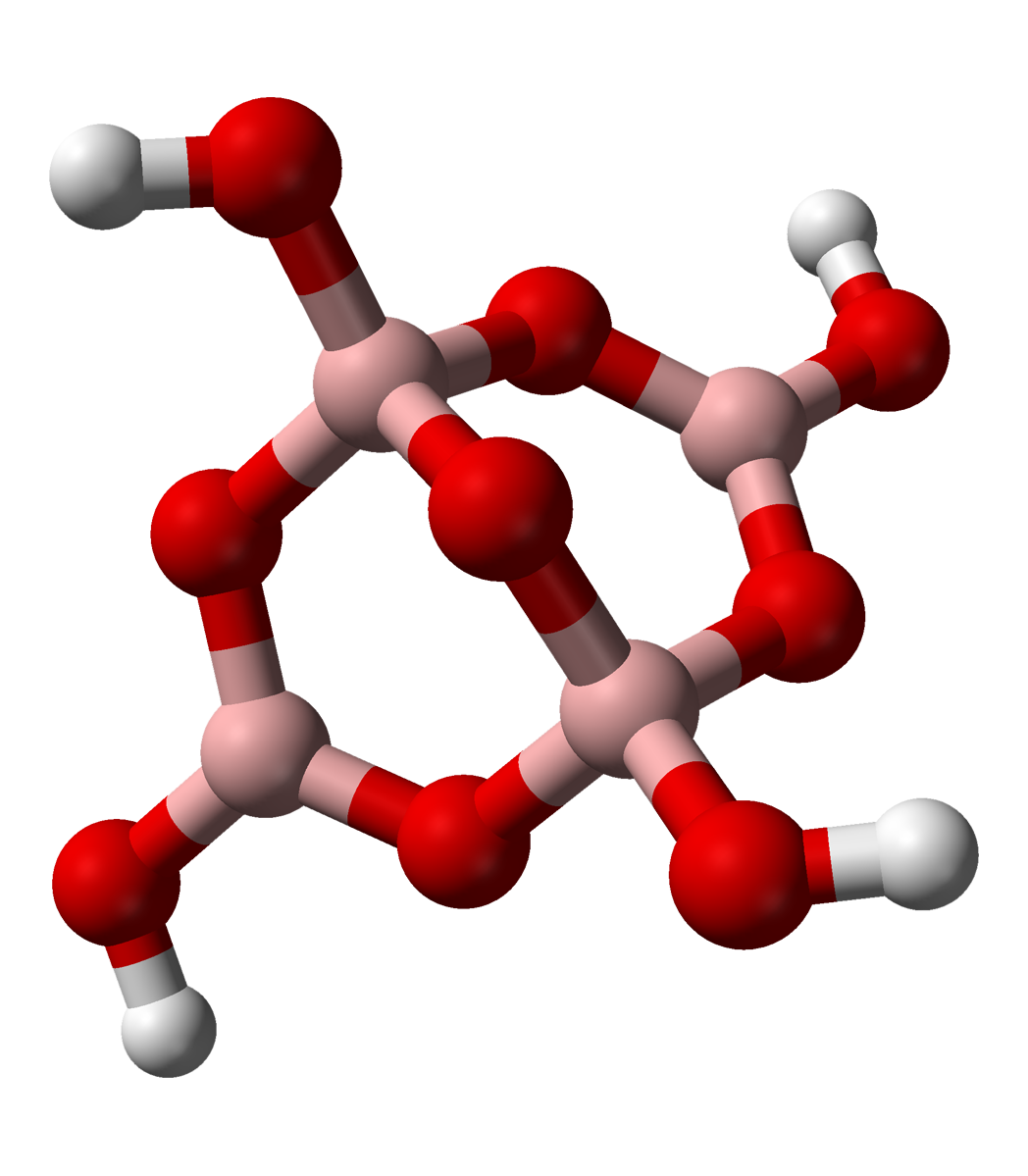
The tetraborate anion (tetramer
A tetramer () ('' tetra-'', "four" + '' -mer'', "parts") is an oligomer formed from four monomers or subunits. The associated property is called ''tetramery''. An example from inorganic chemistry is titanium methoxide with the empirical formula ...
) includes two tetrahedral and two trigonal boron atoms symmetrically assembled in a fused bicyclic structure. The two tetrahedral boron atoms are linked together by a common oxygen atom, and each also bears a negative net charge brought by the supplementary OH groups laterally attached to them. This intricate molecular anion also exhibits three rings: two fused distorted hexagonal (boroxole) rings and one distorted octagonal ring. Each ring is made of a succession of alternate boron and oxygen atoms. Boroxole rings are a very common structural motif in polyborate ions.
The hydrated tetraborate anion occurs in the mineral borax
Borax is a salt (ionic compound), a hydrated borate of sodium, with chemical formula often written . It is a colorless crystalline solid, that dissolves in water to make a basic solution. It is commonly available in powder or granular form ...
(sodium tetraborate octahydrate) with the formula Na2 4O5(OH)4À8H2O. The borax chemical formula is also commonly written in a more compact notation as Na2B4O7┬À10H2O. Sodium borate can be obtained in high purity and so can be used to make a standard solution in titrimetric analysis..
References
Anions Salts Sodium Borates {{chem-stub