|
Terne
Terne plate is a form of tinplate: a thin steel sheet coated with an alloy of lead and tin. The terne alloy was in the ratio of 10-20% tin and the remainder lead. The low tin content made it cheaper than other tinplates. Terne plate was used for tinsmithed sheet metal goods, such as storage vessels, jugs and funnels, particularly for industrial use with flammable liquids. Unlike tinplate, it was not used for long-term storage or around food items, owing to the high lead content. Terne plate has also been used for roofing, as a cheaper alternative to zinc or lead. Until 2012 lead had been replaced with the metal zinc and was used in the ratio of 50% tin and 50% zinc. This alloy had a low melting point of approximately 360 degrees Fahrenheit but is no longer available. Today terne coated metal is coated with 99.9% tin, instead of hot-dipping, a more consistent galvanic deposition process is applied. Additionally the substrate has been changed from steel to stainless steel, benefit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tinning
Tinning is the process of thinly coating sheets of wrought iron or steel with tin, and the resulting product is known as tinplate. The term is also widely used for the different process of coating a metal with solder before soldering. It is most often used to prevent rust, but is also commonly applied to the ends of stranded wire used as electrical conductors to prevent oxidation (which increases electrical resistance), and to keep them from fraying or unraveling when used in various wire connectors like twist-ons, binding posts, or terminal blocks, where stray strands can cause a short circuit. While once more widely used, the primary use of tinplate now is the manufacture of tin cans. Formerly, tinplate was used for cheap pots, pans, and other holloware. This kind of holloware was also known as tinware and the people who made it were tinplate workers. The untinned sheets employed in the manufacture are known as black plates. They are now made of steel, either Bessemer stee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tinplate
Tinplate consists of sheets of steel coated with a thin layer of tin to impede rusting. Before the advent of cheap milled steel, the backing metal was wrought iron. While once more widely used, the primary use of tinplate now is the manufacture of tin cans. Tinplate is made by rolling the steel (or formerly iron) in a rolling mill, removing any mill scale by pickling it in acid and then coating it with a thin layer of tin. Plates were once produced individually (or in small groups) in what became known as a ''pack mill''. In the late 1920s pack mills began to be replaced by ''strip mills'' which produced larger quantities more economically. Formerly, tinplate was used for cheap pots, pans and other holloware. This kind of holloware was also known as tinware and the people who made it were tinplate workers. For many purposes, tinplate has been replaced by galvanised (zinc-coated or tinned) vessels, though not for cooking as zinc is poisonous. The zinc layer prevents the iron f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
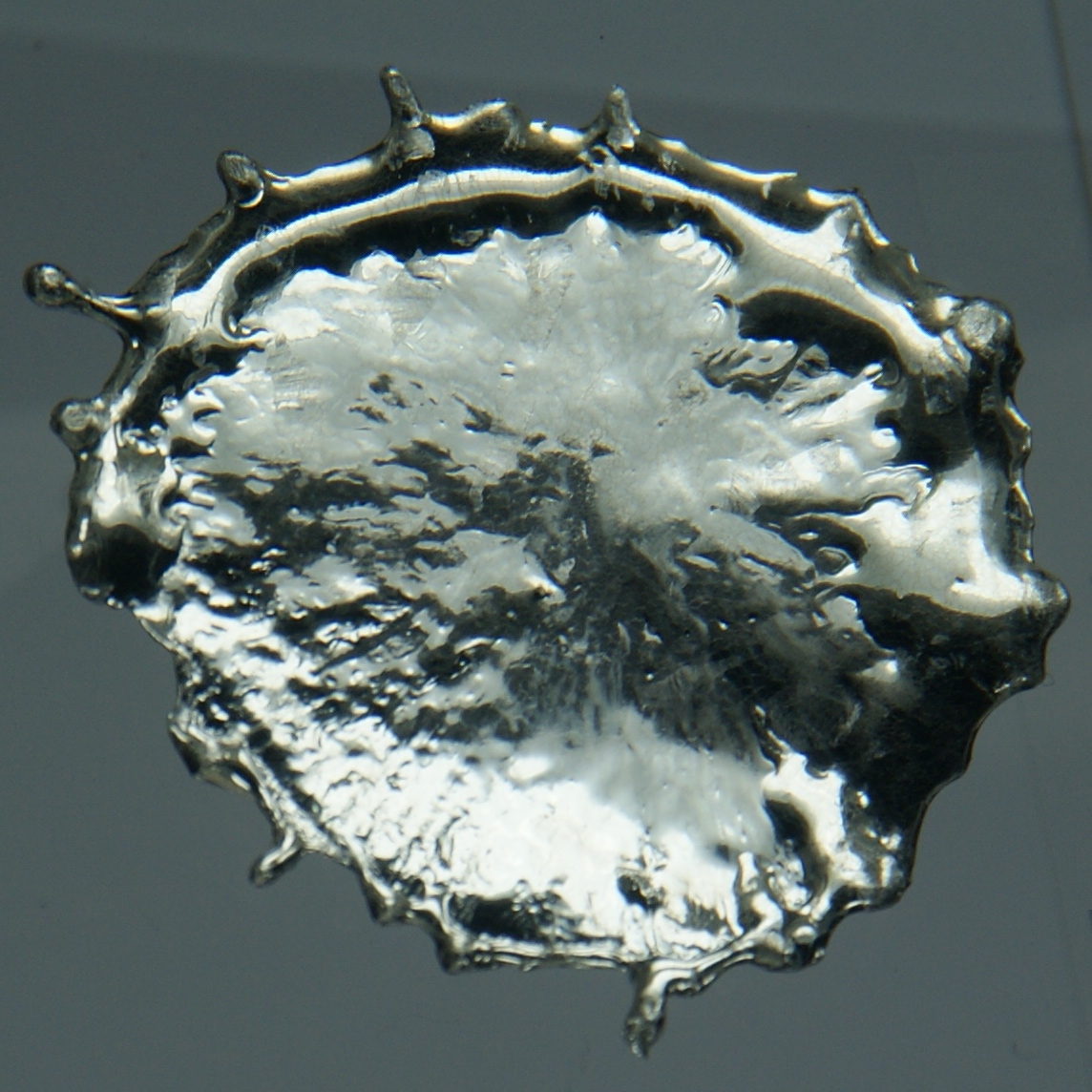
Tin Pest
Tin is a chemical element with the symbol Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal. Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, the so-called "tin cry" can be heard as a result of twinning in tin crystals; this trait is shared by indium, cadmium, zinc, and mercury in the solid state. Pure tin after solidifying presents a mirror-like appearance similar to most metals. In most tin alloys (such as pewter) the metal solidifies with a dull gray color. Tin is a post-transition metal in group 14 of the periodic table of elements. It is obtained chiefly from the mineral cassiterite, which contains stannic oxide, . Tin shows a chemical similarity to both of its neighbors in group 14, germanium and lead, and has two main oxidation states, +2 and the slightly more stable +4. Tin is the 49th most abundant element on Earth and has, with 10 stable isotopes, the largest n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant typically need an additional 11% chromium. Because of its high tensile strength and low cost, steel is used in buildings, infrastructure, tools, ships, trains, cars, machines, electrical appliances, weapons, and rockets. Iron is the base metal of steel. Depending on the temperature, it can take two crystalline forms (allotropic forms): body-centred cubic and face-centred cubic. The interaction of the allotropes of iron with the alloying elements, primarily carbon, gives steel and cast iron their range of unique properties. In pure iron, the crystal structure has relatively little resistance to the iron atoms slipping past one another, and so pure iron is quite ductile, or soft and easily formed. In steel, small amounts of carbon, other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable element and three of its isotopes are endpoints of major nuclear decay chains of heavier elements. Lead is toxic, even in small amounts, especially to children. Lead is a relatively unreactive post-transition metal. Its weak metallic character is illustrated by its amphoteric nature; lead and lead oxides react with acids and bases, and it tends to form covalent bonds. Compounds of lead are usually found in the +2 oxidation state rather than the +4 state common with lighter members of the carbon group. Exceptions are mostly limited to organolead compounds. Like the lighter members of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tinsmith
A tinsmith is a person who makes and repairs things made of tin or other light metals. The profession may sometimes also be known as a tinner, tinker, tinman, or tinplate worker; whitesmith may also refer to this profession, though the same word may also refer to an unrelated specialty of iron-smithing. By extension it can also refer to the person who deals in tinware, or tin plate. Tinsmith was a common occupation in pre-industrial times. Unlike blacksmiths (who work mostly with hot metals), tinsmiths do the majority of their work on cold metal (although they might use a hearth to heat and help shape their raw materials). Tinsmiths fabricate items such as water pitchers, forks, spoons, and candle holders. Training of tinsmiths The tinsmith learned his trade, like many other artisans, by serving an apprenticeship of 4 to 6 years with a master tinsmith. Apprenticeships were considered "indentures" and an apprentice would start first with simply cleaning the shop, polishing t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Funnel
A funnel is a tube or pipe that is wide at the top and narrow at the bottom, used for guiding liquid or powder into a small opening. Funnels are usually made of stainless steel, aluminium, glass, or plastic. The material used in its construction should be sturdy enough to withstand the weight of the substance being transferred, and it should not react with the substance. For this reason, stainless steel or glass are useful in transferring diesel, while plastic funnels are useful in the kitchen. Sometimes disposable paper funnels are used in cases where it would be difficult to adequately clean the funnel afterwards (for example, in adding motor oil into a car). Dropper funnels, also called dropping funnels or tap funnels, have a tap to allow the controlled release of a liquid. A flat funnel, made of polypropylene, utilises living hinges and flexible walls to fold flat. The term "funnel" may refer to the chimney or smokestack on a steam locomotive and commonly refers to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size.The elements are from different metal groups. See periodic table. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity ( electrowinning). Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion. In the most common use of the word, this means electrochemical oxidation of metal in reaction with an oxidant such as oxygen, hydrogen or hydroxide. Rusting, the formation of iron oxides, is a well-known example of electrochemical corrosion. This type of damage typically produces oxide(s) or salt(s) of the original metal and results in a distinctive orange colouration. Corrosion can also occur in materials other than metals, such as ceramics or polymers, although in this context, the term "degradation" is more common. Corrosion degrades the useful properties of materials and structures including strength, appearance and permeability to liquids and gases. Many structural ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stainless Steel
Stainless steel is an alloy of iron that is resistant to rusting and corrosion. It contains at least 11% chromium and may contain elements such as carbon, other nonmetals and metals to obtain other desired properties. Stainless steel's corrosion resistance, resistance to corrosion results from the chromium, which forms a Passivation (chemistry), passive film that can protect the material and self-healing material, self-heal in the presence of oxygen. The alloy's properties, such as luster and resistance to corrosion, are useful in many applications. Stainless steel can be rolled into Sheet metal, sheets, plates, bars, wire, and tubing. These can be used in cookware, cutlery, surgical instruments, major appliances, vehicles, construction material in large buildings, industrial equipment (e.g., in paper mills, chemical plants, water treatment), and storage tanks and tankers for chemicals and food products. The biological cleanability of stainless steel is superior to both alumi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement. Copper is one of the few metals that can occur in nature in a directly usable metallic form ( native metals). This led to very early human use in several regions, from circa 8000 BC. Thousands of years later, it was the first metal to be smelted from sulfide ores, circa 5000 BC; the first metal to be cast into a shape in a mold, c. 4000 BC; and the first metal to be purposely alloyed with another metal, tin, to create ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropes Of Iron
At atmospheric pressure, three allotropic forms of iron exist, depending on temperature: alpha iron (α-Fe), gamma iron (γ-Fe), and delta iron (δ-Fe). At very high pressure, a fourth form exists, called epsilon iron (ε-Fe). Some controversial experimental evidence suggests the existence of a fifth high-pressure form that is stable at very high pressures and temperatures. The phases of iron at atmospheric pressure are important because of the differences in solubility of carbon, forming different types of steel. The high-pressure phases of iron are important as models for the solid parts of planetary cores. The inner core of the Earth is generally assumed to consist essentially of a crystalline iron-nickel alloy with ε structure. The outer core surrounding the solid inner core is believed to be composed of liquid iron mixed with nickel and trace amounts of lighter elements. Standard pressure allotropes Alpha iron (α-Fe) Below 912 °C (1,674 °F), iron has a bod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |