|
Sodium Tetraphenylborate
Sodium tetraphenylborate is the organic compound with the formula NaB(C6H5)4. It is a salt, wherein the anion consists of four phenyl rings bonded to boron. This white crystalline solid is used to prepare other tetraphenylborate salts, which are often highly soluble in organic solvents. The compound is used in inorganic and organometallic chemistry as a precipitating agent for potassium, ammonium, rubidium, and cesium ions, and some organic nitrogen compounds. Synthesis and structure Sodium tetraphenylborate is synthesized by the reaction between sodium tetrafluoroborate and phenylmagnesium bromide: :NaBF4 + 4 PhMgBr → 2 MgBr2 + 2 MgF2 + NaBPh4 (where Ph = phenyl) A related synthesis involves the use of phenylsodium in place of the Grignard reagent. Unlike smaller counteranions, such as nitrate and the halides, tetraphenylborate confers lipophilicity to its salts. Many analogous tetraarylborates have been synthesized, containing both electron-rich and electron-defici ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a Volatility (chemistry), volatile, Combustibility and flammability, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of Carbohydrate, sugars by yeasts or via Petrochemistry, petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the Chemical synthesis, synthesis of organic compounds, and as a Alcohol fuel, fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylphosphite
Trimethyl phosphite is an organophosphorus compound with the formula P(OCH3)3, often abbreviated P(OMe)3. It is a colorless liquid with a highly pungent odor. It is the simplest phosphite ester and finds used as a ligand in organometallic chemistry and as a reagent in organic synthesis. The molecule features a pyramidal phosphorus(III) center bound to three methoxy groups. Synthesis Trimethyl phosphite is in principle obtainable by methanolysis of phosphorus trichloride, say in the presence of a proton accepting base. This method suffers from numerous side reactions however. The use of sodium methoxide is superior: : Reactions Trimethyl phosphite is susceptible to oxidation to trimethyl phosphate: : It reacts with a catalytic amount of methyl iodide in the Arbuzov reaction to give dimethyl methylphosphonate: :P(OCH3)3 → CH3P(O)(OCH3)2 As a ligand, trimethyl phosphite has a smaller cone angle and better acceptor properties relative to trimethylphosphine. A representati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrakis(3,5-bis(trifluoromethyl)phenyl)borate
Tetrakis ,5-bis(trifluoromethyl)phenylorate is an anion with chemical formula 4B.html" ;"title="sub>4B">sub>4Bsup>−, which is commonly abbreviated as ArF4sup>−, indicating the presence of fluorinated aryl (ArF) groups. It is sometimes referred to as ''Kobayashi's anion'' in honour of Hiroshi Kobayashi who led the team that first synthesised it. More commonly it is affectionately nicknamed "BARF." The BARF ion is also abbreviated BArF24−, to distinguish it from the closely related , C6F5)4Bsup>−. BARF has a tetrahedral geometry around the central boron atom but each of the four surrounding aryl groups is aromatic and planar. The motivation for its preparation was the search for an anion that coordinates more weakly than the then-available ions hexafluorophosphate, tetrafluoroborate, or perchlorate. Salts of this anion are known as solids and in both aqueous and non-aqueous solutions. BARF can be used in catalytic systems where the active site requires an anion which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
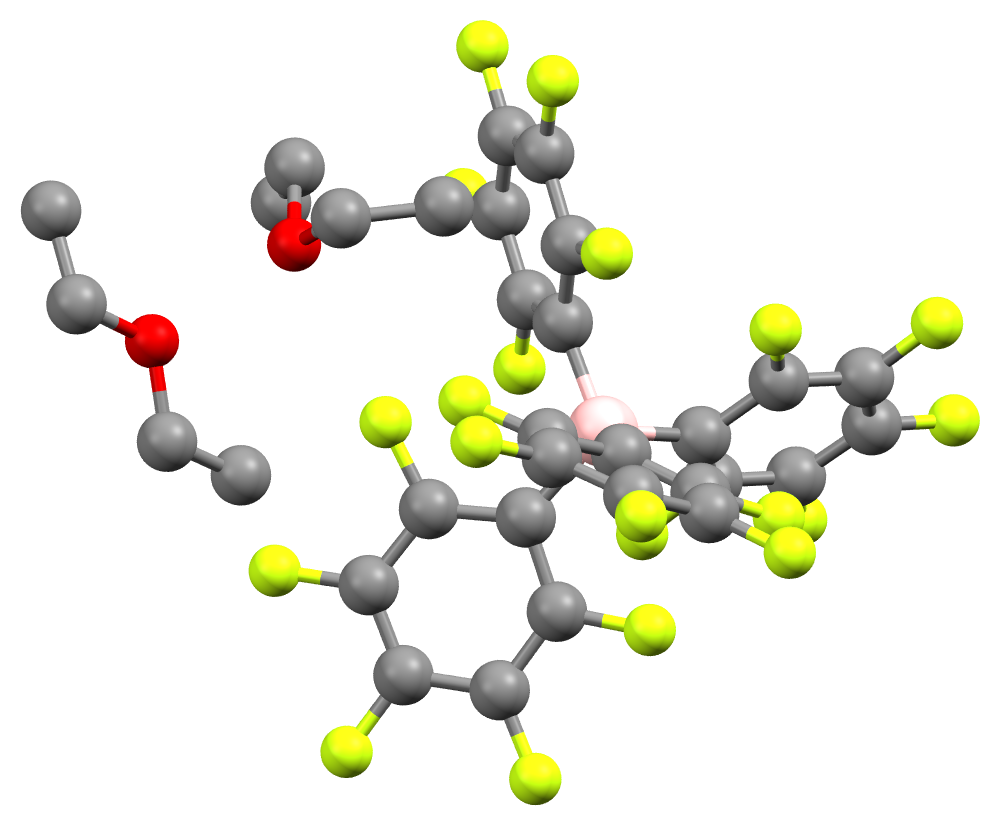
Brookhart's Acid
Brookhart's acid is the salt of the diethyl ether oxonium ion and tetrakis ,5-bis(trifluoromethyl)phenylorate (BAr′4). It is a colorless solid, used as a strong acid. The compound was first reported by Volpe, Grant, and Brookhart in 1992. Preparation This compound is prepared by treatment of NaBAr′4 in diethyl ether (Et2O) with hydrogen chloride: : NaBAr′4 + HCl + 2 Et2O → (OEt2)2sup>+ + NaCl NaBAr′4 is soluble in diethyl ether, whereas sodium chloride is not. Precipitation of sodium chloride thus drives the formation of the oxonium acid compound, which is isolable as a solid. Structure and properties The acid crystallizes as a white, hygroscopic crystalline solid. NMR and elemental analysis showed that the crystal contains two equivalents of diethyl ether. In solution, the compound slowly degrades to ''m''-C6H3(CF3)2 and BAr′3. (OEt2)2B(C6F5)4] is a related compound with a slightly different weakly coordinating anion; it was first reported in 2000. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Weakly Coordinating Anion
Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic cations. They are commonly found as counterions for cationic metal complexes with an 18-Electron rule, unsaturated coordination sphere. These special anions are essential components of Homogeneous catalysis, homogeneous Ziegler–Natta catalyst, alkene polymerisation catalysts, where the active catalyst is a coordinatively unsaturated, cationic transition metal complex. For example, they are employed as counterions for the electron counting, 14 valence electron cations [(C5H5)2ZrR]+ (R = methyl or a growing polyethylene chain). Complexes derived from non-coordinating anions have been used to catalyze hydrogenation, hydrosilylation, oligomerization, and the living polymerization of alkenes. The popularization of non-coordinating anions has contributed to increased underst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structure, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. History Discovery The word "''benzene''" derives from "''gum benzoin''" (benzoin res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylborane
Triphenylborane, often abbreviated to BPh3 where Ph is the phenyl group C6H5-, is a chemical compound with the formula B(C6H5)3. It is a white crystalline solid and is both air and moisture sensitive, slowly forming benzene and triphenylboroxine. It is soluble in aromatic solvents. Structure and properties The core of the compound, BC3, has a trigonal planar structure. The phenyl groups are rotated at about a 30° angle from the core plane. Even though triphenylborane and tris(pentafluorophenyl)borane are structurally similar, their Lewis acidity is not. BPh3 is a weak Lewis acid while B(C6F5)3 is a strong Lewis acid due to the electronegativity of the fluorine atoms. Other boron Lewis acids include BF3 and BCl3. Synthesis Triphenylborane was first synthesized in 1922. It is typically made with boron trifluoride diethyl etherate and the Grignard reagent, phenylmagnesium bromide. : BF3•O(C2H5)2 + 3 C6H5MgBr → B(C6H5)3 + 3 MgBrF + (C2H5)2O Triphenylborane can al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protonolysis
Protonolysis is the cleavage of a chemical bond by acids. Many examples are found in organometallic chemistry since the reaction requires polar Mδ+-Rδ- bonds, where δ+ and δ- signify partial positive and negative charges associated with the bonding atoms. When compounds containing these bonds are treated with acid (HX), these bonds cleave: :M-R + HX → M-X + H-R Hydrolysis (X− = OH−) is a special case of protonolysis. Compounds susceptible to hydrolysis often undergo protonolysis. Hydrides The borohydride anion is susceptible to reaction with even weak acids, resulting protonolysis of one or more B-H bonds. Protonolysis of sodium borohydride with acetic acid gives triacetoxyborohydride: :NaBH4 + 3 HO2CCH3 → NaBH(O2CCH3)3 + 3 H2 Related reactions occur for hydrides of other electropositive elements, e.g. lithium aluminium hydride. Alkyls The alkyl derivatives of many metals undergo protonolysis. For the alkyls of very electropositive metals (zinc, magnesium, and lithium), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dinitrogen Complex
Transition metal dinitrogen complexes are coordination compounds that contain transition metals as ion centers the dinitrogen molecules (N2) as ligands. Historical background Transition metal complexes of N2 have been studied since 1965 when the first complex was reported by Allen and Senoff. This diamagnetic complex, 2+.html" ;"title="u(NH3)5(N2)sup>2+">u(NH3)5(N2)sup>2+, was synthesized from hydrazine hydrate and ruthenium trichloride and consists of a u(NH3)5sup>2+ centre attached to one end of N2. The existence of N2 as a ligand in this compound was identified by IR spectrum with a strong band around 2170–2100 cm−1. In 1966, the molecular structure of u(NH3)5(N2)l2 was determined by Bottomly and Nyburg by X-ray crystallography. The dinitrogen complex ''trans''- rCl(N2)(PPh3)2is made by treating Vaska's complex with aromatic acyl azides. It has a planar geometry. The first preparation of a metal-dinitrogen complex using dinitrogen was reported in 1967 by Yam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steven Ittel
Steven Dale Ittel (born 1946 in Hamilton, Ohio) is an American chemist specializing in organometallic chemistry and homogeneous catalysis. Training Ittel attended Miami University in Oxford, Ohio, where he received a bachelor's degree in chemistry in 1968. He was then commissioned as an officer in the United States Public Health Service and studied photochemical smog in the New York City metropolitan area from 1968 to 1970. He attended Northwestern University, where he received his PhD in chemistry under the direction of James A. Ibers in 1974. Career Ittel worked on hydride activation of lanthanides for Systems for Nuclear Auxiliary Power (SNAP) at Monsanto's Mound Laboratories for a short time. Upon receiving his PhD from Northwestern University, he joined DuPont’s Central Research Department at the Experimental Station in Wilmington, Delaware. Ittel is best known for his contributions to organometallic chemistry and homogeneous catalysis. He discovered fluxional processes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not classed as organic). It is produced mainly as a byproduct of acrylonitrile manufacture. It is used as a polar aprotic solvent in organic synthesis and in the purification of butadiene. The skeleton is linear with a short distance of 1.16 Å. Acetonitrile was first prepared in 1847 by the French chemist Jean-Baptiste Dumas. Applications Acetonitrile is used mainly as a solvent in the purification of butadiene in refineries. Specifically, acetonitrile is fed into the top of a distillation column filled with hydrocarbons including butadiene, and as the acetonitrile falls down through the column, it absorbs the butadiene which is then sent from the bottom of the tower to a second separating tower. Heat is then employed in the separatin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

4.png)
2.png)
