|
Phosphate Buffered Saline
Phosphate-buffered saline (PBS) is a buffer solution (pH ~ 7.4) commonly used in biological research. It is a water-based salt solution containing disodium hydrogen phosphate, sodium chloride and, in some formulations, potassium chloride and potassium dihydrogen phosphate. The buffer helps to maintain a constant pH. The osmolarity and ion concentrations of the solutions match those of the human body ( isotonic). Applications PBS has many uses because it is isotonic and non-toxic to most cells. These uses include substance dilution and cell container rinsing. PBS with EDTA is also used to disengage attached and clumped cells. Divalent metals such as zinc, however, cannot be added as this will result in precipitation. For these types of applications, Good's buffers are recommended. PBS has been shown to be an acceptable alternative to viral transport medium regarding transport and storage of RNA viruses, such as SARS-CoV-2 Preparation There are many different ways to prepare PBS so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buffer Solution
A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature, there are many living systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also acts as a buffer in the ocean. Principles of buffering Buffer solutions resist pH change because of a chemical equilibrium between the weak acid HA and its conjugate base A−: When some strong acid is added to an equilibrium mixture of the weak acid and its conjugate base, hydrogen ions (H+) are added, and the equilibrium is shifted to the left, in accordance with Le Chatelier's principle. Because of this, the hydrogen io ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Chloride
Potassium chloride (KCl, or potassium salt) is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water, and its solutions have a salt-like taste. Potassium chloride can be obtained from ancient dried lake deposits. KCl is used as a fertilizer, in medicine, in scientific applications, domestic water softeners (as a substitute for sodium chloride salt), and in food processing, where it may be known as E number additive E508. It occurs naturally as the mineral sylvite, and in combination with sodium chloride as sylvinite. Uses Fertilizer The majority of the potassium chloride produced is used for making fertilizer, called potash, since the growth of many plants is limited by potassium availability. Potassium chloride sold as fertilizer is known as muriate of potash (MOP). The vast majority of potash fertilizer worldwide is sold as MOP. Medical use Potassium is vital ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and metabolism. Over the last decades of the 20th century, biochemistry has become successful at explaining living processes through these three disciplines. Almost all areas of the life sciences are being uncovered and developed through biochemical methodology and research. Voet (2005), p. 3. Biochemistry focuses on understanding the chemical basis which allows biological molecules to give rise to the processes that occur within living cells and between cells,Karp (2009), p. 2. in turn relating greatly to the understanding of tissues and organs, as well as organism structure and function.Miller (2012). p. 62. Biochemistry is closely related to molecular biology, which is the study of the molecular mechanisms of biological phenomena.As ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buffer Solutions
A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature, there are many living systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also acts as a buffer in the ocean. Principles of buffering Buffer solutions resist pH change because of a chemical equilibrium between the weak acid HA and its conjugate base A−: When some strong acid is added to an equilibrium mixture of the weak acid and its conjugate base, hydrogen ions (H+) are added, and the equilibrium is shifted to the left, in accordance with Le Chatelier's principle. Because of this, the hydrogen ion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brine
Brine is a high-concentration solution of salt (NaCl) in water (H2O). In diverse contexts, ''brine'' may refer to the salt solutions ranging from about 3.5% (a typical concentration of seawater, on the lower end of that of solutions used for brining foods) up to about 26% (a typical saturated solution, depending on temperature). Brine forms naturally due to evaporation of ground saline water but it is also generated in the mining of sodium chloride. Brine is used for food processing and cooking (pickling and brining), for de-icing of roads and other structures, and in a number of technological processes. It is also a by-product of many industrial processes, such as desalination, so it requires wastewater treatment for proper disposal or further utilization (fresh water recovery). In nature Brines are produced in multiple ways in nature. Modification of seawater via evaporation results in the concentration of salts in the residual fluid, a characteristic geologic deposit call ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tris-buffered Saline
Tris-buffered saline (TBS) is a buffer used in some biochemical techniques to maintain the pH within a relatively narrow range. Tris Tris, or tris(hydroxymethyl)aminomethane, or known during medical use as tromethamine or THAM, is an organic compound with the formula (HOCH2)3CNH2, one of the twenty Good's buffers. It is extensively used in biochemistry and molecular biology as ... (with HCl) has a slightly alkaline buffering capacity in the 7–9.2 range. The conjugate acid of Tris has a pKa of 8.07 at 25 °C. The pKa declines approximately 0.03 units per degree Celsius rise in temperature. This can lead to relatively dramatic pH shifts when there are shifts in solution temperature. Sodium chloride concentration may vary from 100 to 200 mM, tris concentration from 5 to 100 mM and pH from 7.2 to 8.0. A common formulation of TBS is 150 mM NaCl, 50 mM Tris-HCl, pH 7.6. TBS can also be prepared by using commercially made TBS buffer tablets or pouches. Applications TBS is isoton ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borate Buffered Saline
Borate buffered saline (abbreviated BBS) is a buffer used in some biochemical techniques to maintain the pH within a relatively narrow range. Borate buffers have an alkaline buffering capacity in the 8–10 range. Boric acid has a pKa of 9.14 at 25°C. Applications BBS has many uses because it is isotonic and has a strong bactericidal effect. It can be used to dilute substances and has applications in coating procedures. Additives such as olysorbate 20and milk powder can be used to add to BBS's functionality as a washing buffer or blocking buffer. Contents The following is a sample recipe for BBS: *10 mM Sodium borate *150 mM NaCl Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ... Adjust pH to pH 8.2 The simplest way to prepare a BBS solution is to use BBS tablets. They are for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Precipitate
In an aqueous solution, precipitation is the process of transforming a dissolved substance into an insoluble solid from a super-saturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the ''precipitant''. The clear liquid remaining above the precipitated or the centrifuged solid phase is also called the 'supernate' or 'supernatant'. The notion of precipitation can also be extended to other domains of chemistry (organic chemistry and biochemistry) and even be applied to the solid phases (''e.g.'', metallurgy and alloys) when solid impurities segregate from a solid phase. Supersaturation The precipitation of a compound may occur when its concentration exceeds its solubility. This can be due to temperature changes, solvent evaporation, or by mixing solvents. Precipitation occurs more rapidly from a strongly supersaturated solution. The formati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Filtration
Filtration is a physical separation process that separates solid matter and fluid from a mixture using a ''filter medium'' that has a complex structure through which only the fluid can pass. Solid particles that cannot pass through the filter medium are described as ''oversize'' and the fluid that passes through is called the ''filtrate''. Oversize particles may form a filter cake on top of the filter and may also block the filter lattice, preventing the fluid phase from crossing the filter, known as ''blinding''. The size of the largest particles that can successfully pass through a filter is called the effective ''pore size'' of that filter. The separation of solid and fluid is imperfect; solids will be contaminated with some fluid and filtrate will contain fine particles (depending on the pore size, filter thickness and biological activity). Filtration occurs both in nature and in engineered systems; there are biological, geological, and industrial forms. Filtration is als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
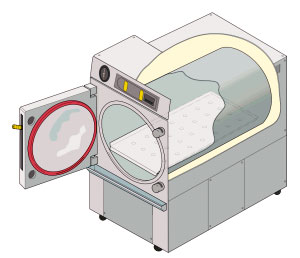
Autoclaving
An autoclave is a machine used to carry out industrial and scientific processes requiring elevated temperature and pressure in relation to ambient pressure and/or temperature. Autoclaves are used before surgical procedures to perform sterilization and in the chemical industry to cure coatings and vulcanize rubber and for hydrothermal synthesis. Industrial autoclaves are used in industrial applications, especially in the manufacturing of composites. Many autoclaves are used to sterilize equipment and supplies by subjecting them to pressurized saturated steam at for around 30-60 minutes at a pressure of 15 psi (103 kPa or 1.02 atm) depending on the size of the load and the contents. The autoclave was invented by Charles Chamberland in 1879, although a precursor known as the steam digester was created by Denis Papin in 1679. The name comes from Greek ''auto-'', ultimately meaning self, and Latin ''clavis'' meaning key, thus a self-locking device. Uses Sterilization autoclav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monopotassium Phosphate
Monopotassium phosphate (MKP) (also, potassium dihydrogenphosphate, KDP, or monobasic potassium phosphate) is the inorganic compound with the formula KH2PO4. Together with dipotassium phosphate (K2HPO4.(H2O)x) it is often used as a fertilizer, food additive, and buffering agent. The salt often cocrystallizes with the dipotassium salt as well as with phosphoric acid. Single crystals are paraelectric at room temperature. At temperatures below , they become ferroelectric. Structure Monopotassium phosphate can exist in several Polymorphism (materials science), polymorphs. At room temperature it forms paraelectric crystals with tetragonal symmetry. Upon cooling to it transforms to a ferroelectric phase of orthorhombic symmetry, and the transition temperature shifts up to when hydrogen is replaced by deuterium. Heating to changes its structure to monoclinic. When heated further, MKP decomposes, by loss of water, to potassium metaphosphate, , at . Manufacturing Monopotassium phos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disodium Phosphate
Disodium phosphate (DSP), or disodium hydrogen phosphate, or sodium phosphate dibasic, is the inorganic compound with the formula Na2HPO4. It is one of several sodium phosphates. The salt is known in anhydrous form as well as forms with 2, 7, 8, and 12 hydrates. All are water-soluble white powders; the anhydrous salt being hygroscopic. The pH of disodium hydrogen phosphate water solution is between 8.0 and 11.0, meaning it is moderately basic: :HPO42− + H2O H2PO4− + OH− Production and reactions It can be generated by neutralization of phosphoric acid with sodium hydroxide: :H3PO4 + 2 NaOH → Na2HPO4 + 2 H2O Industrially It is prepared in a two-step process by treating dicalcium phosphate with sodium bisulfate, which precipitates calcium sulfate:Klaus Schrödter, Gerhard Bettermann, Thomas Staffel, Friedrich Wahl, Thomas Klein, Thomas Hofmann "Phosphoric Acid and Phosphates" in ''Ullmann’s Encyclopedia of Industrial Chemistry'' 2008, Wiley-VCH, Weinhei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |