|
Protein Phosphatases
A protein phosphatase is a phosphatase enzyme that removes a phosphate group from the phosphorylated amino acid residue of its substrate protein. Protein phosphorylation is one of the most common forms of reversible protein posttranslational modification ( PTM), with up to 30% of all proteins being phosphorylated at any given time. Protein kinases (PKs) are the effectors of phosphorylation and catalyse the transfer of a γ-phosphate from ATP to specific amino acids on proteins. Several hundred PKs exist in mammals and are classified into distinct super-families. Proteins are phosphorylated predominantly on Ser, Thr and Tyr residues, which account for 79.3, 16.9 and 3.8% respectively of the phosphoproteome, at least in mammals. In contrast, protein phosphatases (PPs) are the primary effectors of dephosphorylation and can be grouped into three main classes based on sequence, structure and catalytic function. The largest class of PPs is the phosphoprotein phosphatase (PPP) family comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphatase
In biochemistry, a phosphatase is an enzyme that uses water to cleave a phosphoric acid Ester, monoester into a phosphate ion and an Alcohol (chemistry), alcohol. Because a phosphatase enzyme catalysis, catalyzes the hydrolysis of its Substrate (chemistry), substrate, it is a subcategory of hydrolases. Phosphatase enzymes are essential to many biological functions, because phosphorylation (e.g. by protein kinases) and dephosphorylation (by phosphatases) serve diverse roles in cell growth, cellular regulation and cell signaling, signaling. Whereas phosphatases remove phosphate groups from molecules, kinases catalyze the transfer of phosphate groups to molecules from Adenosine triphosphate, ATP. Together, kinases and phosphatases direct a form of post-translational modification that is essential to the cell's regulatory network. Phosphatase enzymes are not to be confused with phosphorylase enzymes, which catalyze the transfer of a phosphate group from hydrogen phosphate to an acce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium where it ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline Phosphatase
The enzyme alkaline phosphatase (EC 3.1.3.1, alkaline phosphomonoesterase; phosphomonoesterase; glycerophosphatase; alkaline phosphohydrolase; alkaline phenyl phosphatase; orthophosphoric-monoester phosphohydrolase (alkaline optimum), systematic name phosphate-monoester phosphohydrolase (alkaline optimum)) catalyses the following reaction: : a phosphate monoester + H2O = an alcohol + phosphate Alkaline phosphatase has the physiological role of dephosphorylating compounds. The enzyme is found across a multitude of organisms, prokaryotes and eukaryotes alike, with the same general function but in different structural forms suitable to the environment they function in. Alkaline phosphatase is found in the periplasmic space of '' E. coli'' bacteria. This enzyme is heat stable and has its maximum activity at high pH. In humans, it is found in many forms depending on its origin within the body – it plays an integral role in metabolism within the liver and development withi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
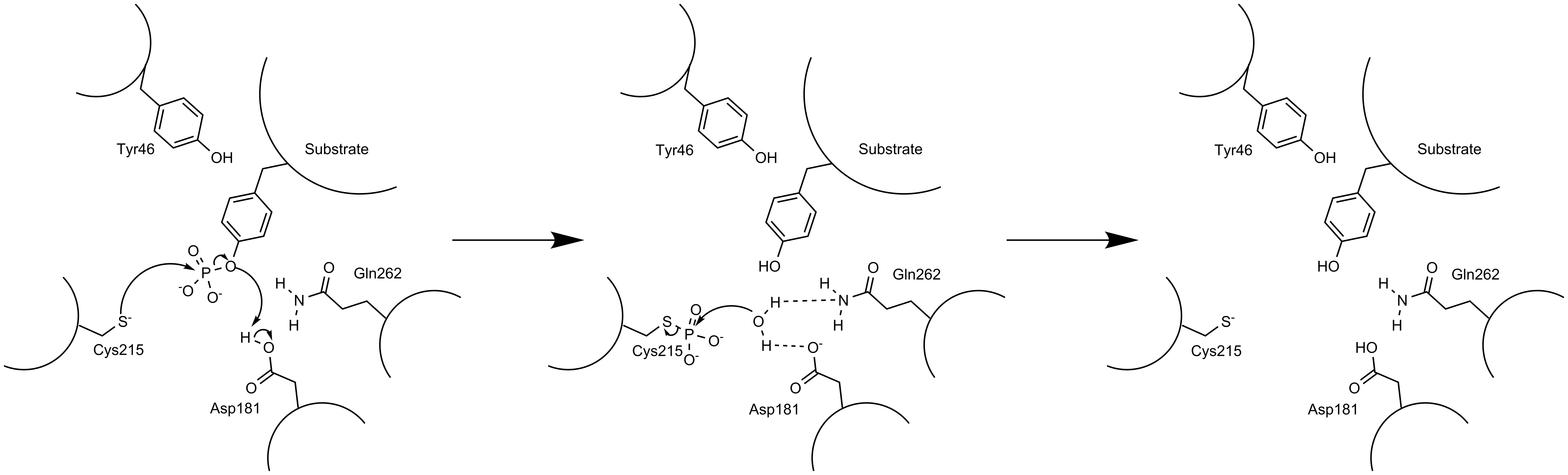
Protein Tyrosine Phosphatase
Protein tyrosine phosphatases (EC 3.1.3.48, systematic name protein-tyrosine-phosphate phosphohydrolase) are a group of enzymes that remove phosphate groups from phosphorylated tyrosine residues on proteins: : proteintyrosine phosphate + H2O = proteintyrosine + phosphate Protein tyrosine (pTyr) phosphorylation is a common post-translational modification that can create novel recognition motifs for protein interactions and cellular localization, affect protein stability, and regulate enzyme activity. As a consequence, maintaining an appropriate level of protein tyrosine phosphorylation is essential for many cellular functions. Tyrosine-specific protein phosphatases (PTPase; ) catalyse the removal of a phosphate group attached to a tyrosine residue, using a cysteinyl-phosphate enzyme intermediate. These enzymes are key regulatory components in signal transduction pathways (such as the MAP kinase pathway) and cell cycle control, and are important in the control of cell growth, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the deprotonated –COO− form under biological conditions), and an imidazole side chain (which is partially protonated), classifying it as a positively charged amino acid at physiological pH. Initially thought essential only for infants, it has now been shown in longer-term studies to be essential for adults also. It is encoded by the codons CAU and CAC. Histidine was first isolated by Albrecht Kossel and Sven Gustaf Hedin in 1896. It is also a precursor to histamine, a vital inflammatory agent in immune responses. The acyl radical is histidyl. Properties of the imidazole side chain The conjugate acid (protonated form) of the imidazole side chain in histidine has a p''K''a of approximately 6.0. Thus, below a pH of 6, the imidazole ring ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DUSP28
Dual-specificity phosphatase (DUSP; DSP) is a form of phosphatase that can act upon tyrosine or serine/threonine residues. There are several families of dual-specificity phosphatase enzymes in mammals. All share a similar catalytic mechanism, by which a conserved cysteine residue forms a covalent intermediate with the phosphate group to be eliminated. The residues surrounding their catalytic core obey a rather strict consensus: His-Cys-x-x-x-x-x-Arg-Ser. The serine side chain and an additional conserved aspartate play a central role in the elimination of the Cys-linked intermediate, thus completing their enzymatic cycle. The main difference between tyrosine-specific phosphatases and dual-specificity phosphatases lies in the width of the latter enzymes' catalytic pocket: thus they can accommodate phosphorylated serine or threonine side chains as well as phosphorylated tyrosines. Classification The human genome encodes at least 61 different DUSP proteins. The following major groups ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DUSP1
Dual specificity protein phosphatase 1 is an enzyme that in humans is encoded by the ''DUSP1'' gene. Function The expression of DUSP1 gene is induced in human skin fibroblasts by oxidative/heat stress and growth factors. It specifies a protein with structural features similar to members of the non-receptor-type protein-tyrosine phosphatase family, and which has significant amino-acid sequence similarity to a Tyr/Ser-protein phosphatase encoded by the late gene H1 of vaccinia virus. The bacterially expressed and purified DUSP1 protein has intrinsic phosphatase activity, and specifically inactivates mitogen-activated protein (MAP) kinase in vitro by the concomitant dephosphorylation of both its phosphothreonine and phosphotyrosine residues. Furthermore, it suppresses the activation of MAP kinase by oncogenic ras in extracts of Xenopus oocytes. Thus, DUSP1 may play an important role in the human cellular response to environmental stress as well as in the negative regulation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PPP2CA
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform is an enzyme that (in humans) is encoded by the ''PPP2CA'' gene. Function This gene encodes the phosphatase 2A catalytic subunit. Protein phosphatase 2A is one of the four major Ser/Thr phosphatases, and it is implicated in the negative control of cell growth and division. It consists of a common heteromeric core enzyme, which is composed of a catalytic subunit and a constant regulatory subunit, that associates with a variety of regulatory subunits. This gene encodes an alpha isoform of the catalytic subunit. Interactions PPP2CA has been shown to interact with: * Bcl-2, * Bestrophin 1, * CCNG2, * CTTNBP2NL, * CTTNBP2, * Cyclin-dependent kinase 2, * Cyclin-dependent kinase 6, * FAM40A, * IGBP1, * MOBKL3, * PPP2R1A, * PPP2R1B, * PPP2R2A, * PPP2R3B, * PPP2R5A, * PPP2R5B, * PPP2R5C, * PPP2R5D, * PPP2R5E, * STRN3, * STRN, and * TLX1. See also *PPP2CB Serine/threonine-protein ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), a carboxyl group (which is in the deprotonated −COO− form under biological conditions), and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as ''E. coli''. It is encoded by all the codons starting AC (ACU, ACC, ACA, and ACG). Threonine sidechains are often hydrogen bonded; the most common small motifs formed are based on interactions with serine: ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices). Modifications The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can unde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form under biological conditions), and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC. Occurrence This compound is one of the naturally occurring proteinogenic amino acids. Only the L-stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine. Serine was first obtained from silk protein, a particularly rich source, in 1865 by Emil Cramer. Its name is derived from the Latin for silk, ''sericum''. Serine's structure was estab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PTP1B
Tyrosine-protein phosphatase non-receptor type 1 also known as protein-tyrosine phosphatase 1B (PTP1B) is an enzyme that is the founding member of the protein tyrosine phosphatase (PTP) family. In humans it is encoded by the ''PTPN1'' gene. PTP1B is a negative regulator of the insulin signaling pathway and is considered a promising potential therapeutic target, in particular for treatment of type 2 diabetes. It has also been implicated in the development of breast cancer and has been explored as a potential therapeutic target in that avenue as well. Structure and function PTP1B was first isolated from a human placental protein extract, but it is expressed in many tissues. PTP1B is localized to the cytoplasmic face of the endoplasmic reticulum. PTP1B can dephosphorylate the phosphotyrosine residues of the activated insulin receptor kinase. In mice, genetic ablation of PTPN1 results in enhanced insulin sensitivity. Several other tyrosine kinases, including epidermal growth factor r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek ''tyrós'', meaning ''cheese'', as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a Hydrophobe, hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is Genetic code, encoded by the Genetic code#Codons, codons UAC and UAU in messenger RNA. Functions Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of the phenol functionality. It occurs in proteins that are part of signal transduction processes and functions as a receiver of phosphate groups that are transferred by way of protein kinases. Phosphorylation of the hyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |