|
Photomethionine
L-Photo-methionine is a photo-reactive amino acid derivative of L-methionine that was synthetically formed in 2005. Protein are long polymer chains of amino acids; which can range in various structures and sizes. Proteins can interact with each other ( protein-protein interactions or PPI) and with these interactions, affects cellular interactions and pathways. Such interactions; in viral fusion and in growth-factor signaling looked promising for antiviral or anti-cancer drugs, so research must be done to understand the interactions. With that, research has begun to prove that proteins function in supramolecular complexes compared to isolated entities. So, scientists Monika Suchanek, Anna Radzikowski, and Christoph Thiele researched that the direct way to study these interactions in the natural environment better was to create a new way of photo-cross-linking proteins; which led to the synthesis of L-photo-methionine and in that same study, L-photo-leucine. Synthesis Racemic Ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
L-methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical role in the metabolism and health of many species, including humans. It is Genetic code, encoded by the codon AUG. Methionine is also an important part of angiogenesis, the growth of new blood vessels. Supplementation may benefit those suffering from copper poisoning. Overconsumption of methionine, the methyl group donor in DNA methylation, is related to cancer growth in a number of studies. Methionine was first isolated in 1921 by John Howard Mueller. Biochemical details Methionine (abbreviated as Met or M; encoded by the codon AUG) is an α-amino acid that is used in the biosynthesis of proteins. It contains a carboxyl group (which is in the deprotonated −COO− form under biological pH conditions), an amino group (which is in the proton ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mothers Against Decapentaplegic Homolog 2
Mothers against decapentaplegic homolog 2 also known as SMAD family member 2 or SMAD2 is a protein that in humans is encoded by the ''SMAD2'' gene. MAD homolog 2 belongs to the SMAD, a family of proteins similar to the gene products of the ''Drosophila'' gene 'mothers against decapentaplegic' (Mad) and the ''C. elegans'' gene Sma. SMAD proteins are signal transducers and transcriptional modulators that mediate multiple signaling pathways. Function SMAD2 mediates the signal of the transforming growth factor (TGF)-beta, and thus regulates multiple cellular processes, such as cell proliferation, apoptosis, and differentiation. This protein is recruited to the TGF-beta receptors through its interaction with the SMAD anchor for receptor activation (SARA) protein. In response to TGF-beta signal, this protein is phosphorylated by the TGF-beta receptors. The phosphorylation induces the dissociation of this protein with SARA and the association with the family member SMAD4. The ass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Progesterone
Progesterone (P4) is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species. It belongs to a group of steroid hormones called the progestogens and is the major progestogen in the body. Progesterone has a variety of important functions in the body. It is also a crucial metabolic intermediate in the production of other endogenous steroids, including the sex hormones and the corticosteroids, and plays an important role in brain function as a neurosteroid. In addition to its role as a natural hormone, progesterone is also used as a medication, such as in combination with estrogen for contraception, to reduce the risk of uterine or cervical cancer, in hormone replacement therapy, and in feminizing hormone therapy. It was first prescribed in 1934. Biological activity Progesterone is the most important progestogen in the body. As a potent agonist of the nuclear progesterone receptor (nPR) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PGRMC1
Progesterone receptor membrane component 1 (abbreviated PGRMC1) is a protein which co-purifies with progesterone binding proteins in the liver and ovary. In humans, the PGRMC1 protein is encoded by the ''PGRMC1'' gene. The sole biochemical function of PGRMC1 is heme-binding. PGRMC1 shares key structural motifs with cytochrome b5. PGRMC1 binds and activates P450 proteins, which are important in drug, hormone and lipid metabolism. PGRMC1 also binds to PAIR-BP1 (plasminogen activator inhibitor RNA-binding protein-1). However, its expression outside of the reproductive tract and in males suggests multiple functions for the protein. These may include binding to Insig (insulin-induced gene), which regulates cholesterol synthesis. Expression PGRMC1 is highly expressed in the liver and kidney in humans with lower expression in the brain, lung, heart, skeletal muscle and pancreas. In rodents, PGRMC1 is found in the liver, lung, kidney and brain. PGRMC1 is over-expressed in breast ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
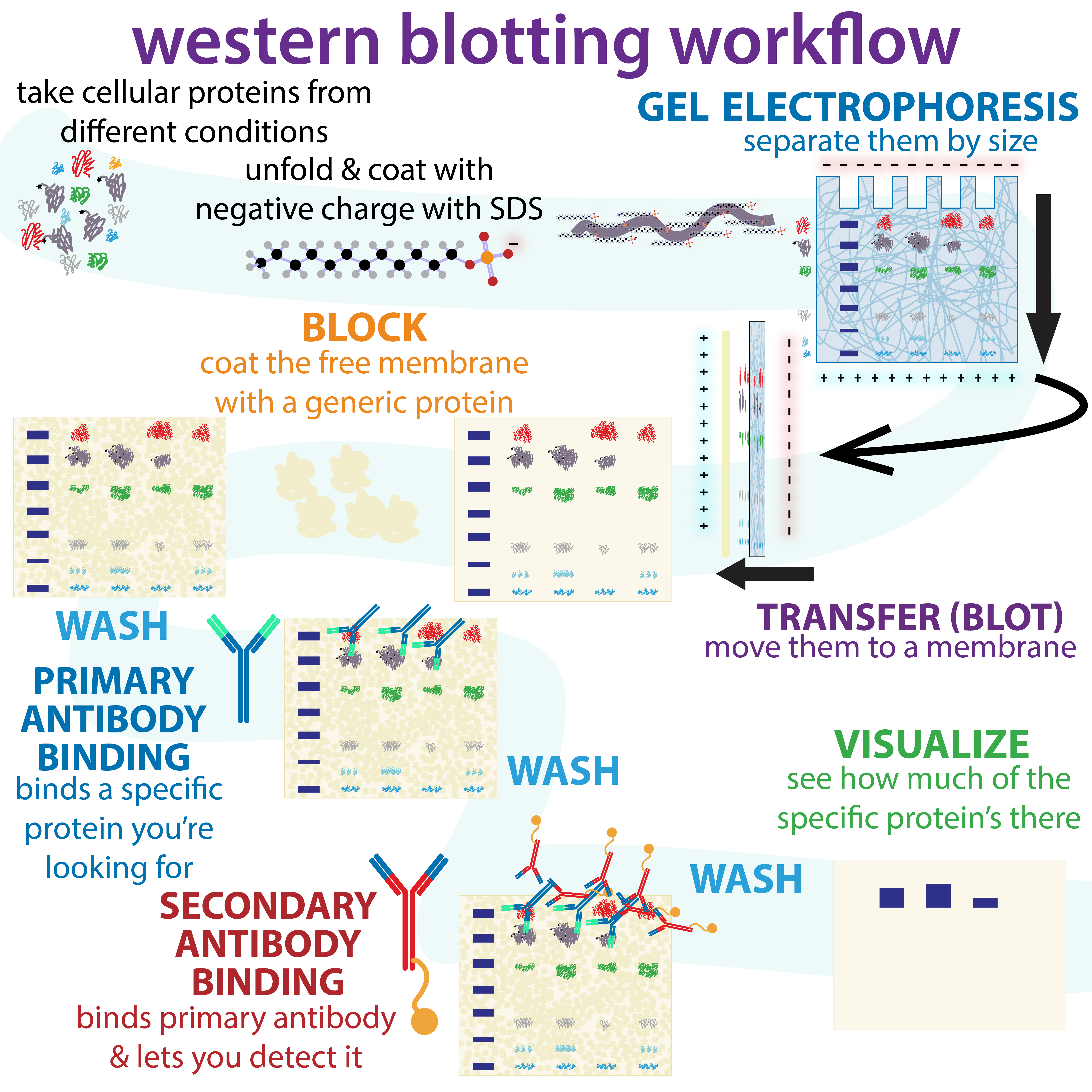
Western Blot
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detecting the proteins, this technique is also utilized to visualize, distinguish, and quantify the different proteins in a complicated protein combination. Western blot technique uses three elements to achieve its task of separating a specific protein from a complex: separation by size, transfer of protein to a solid support, and marking target protein using a primary and secondary antibody to visualize. A synthetic or animal-derived antibody (known as the primary antibody) is created that recognizes and binds to a specific target protein. The electrophoresis membrane is washed in a solution containing the primary antibody, before excess antibody is washed off. A secondary antibody is added which recognizes and binds to the primary antibody ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
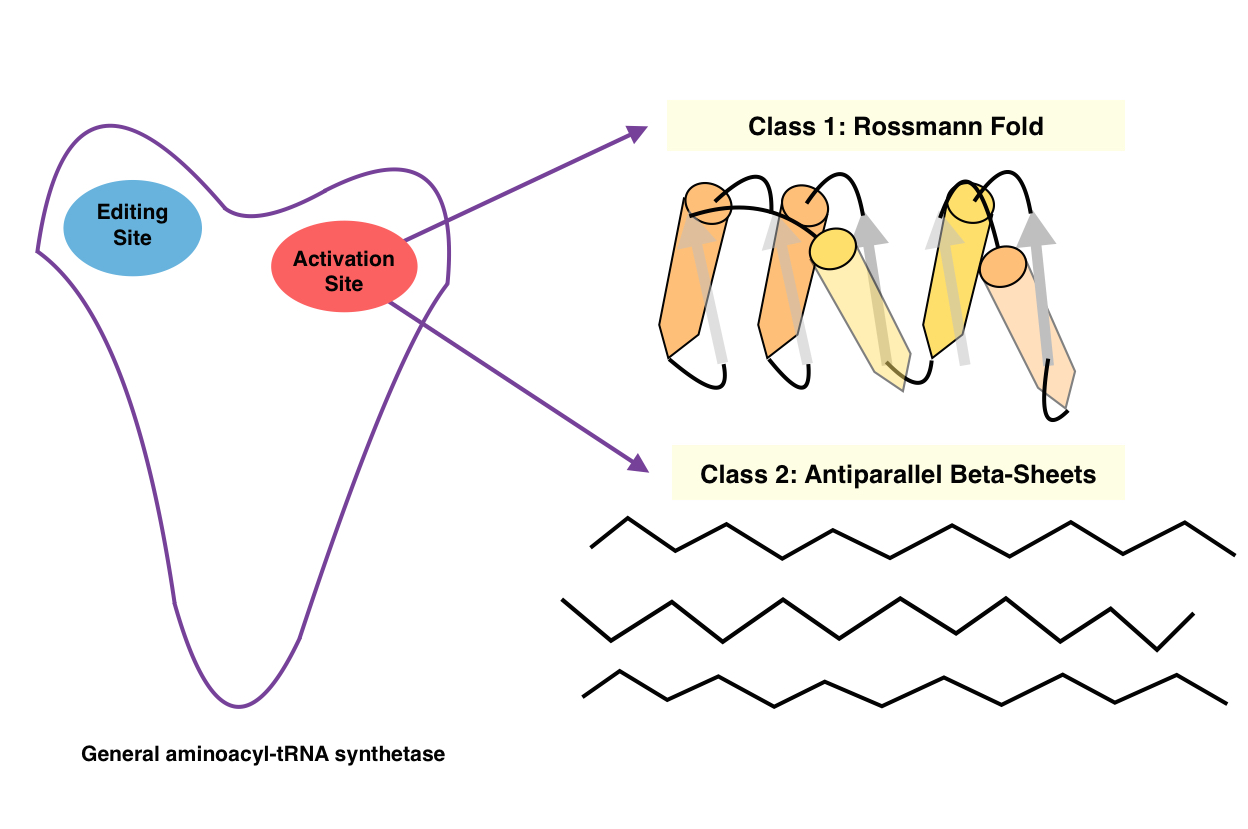
Aminoacyl TRNA Synthetase
An aminoacyl-tRNA synthetase (aaRS or ARS), also called tRNA-ligase, is an enzyme that attaches the appropriate amino acid onto its corresponding tRNA. It does so by catalyzing the transesterification of a specific cognate amino acid or its precursor to one of all its compatible cognate tRNAs to form an aminoacyl-tRNA. In humans, the 20 different types of aa-tRNA are made by the 20 different aminoacyl-tRNA synthetases, one for each amino acid of the genetic code. This is sometimes called "charging" or "loading" the tRNA with an amino acid. Once the tRNA is charged, a ribosome can transfer the amino acid from the tRNA onto a growing peptide, according to the genetic code. Aminoacyl tRNA therefore plays an important role in RNA translation, the expression of genes to create proteins. Mechanism The synthetase first binds ATP and the corresponding amino acid (or its precursor) to form an aminoacyl-adenylate, releasing inorganic pyrophosphate (PPi). The adenylate-aaRS complex then ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transfer RNA
Transfer RNA (abbreviated tRNA and formerly referred to as sRNA, for soluble RNA) is an adaptor molecule composed of RNA, typically 76 to 90 nucleotides in length (in eukaryotes), that serves as the physical link between the mRNA and the amino acid sequence of proteins. tRNAs genes from Bacteria are typically shorter (mean = 77.6 bp) than tRNAs from Archaea (mean = 83.1 bp) and eukaryotes (mean = 84.7 bp). The mature tRNA follows an opposite pattern with tRNAs from Bacteria being usually longer (median = 77.6 nt) than tRNAs from Archaea (median = 76.8 nt), with eukaryotes exhibiting the shortest mature tRNAs (median = 74.5 nt). Transfer RNA (tRNA) does this by carrying an amino acid to the protein synthesizing machinery of a cell called the ribosome. Complementation of a 3-nucleotide codon in a messenger RNA (mRNA) by a 3-nucleotide anticodon of the tRNA results in protein synthesis based on the mRNA code. As such, tRNAs are a necessary component of translation, the biological sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sterol Regulatory Element-binding Protein
Sterol regulatory element-binding proteins (SREBPs) are transcription factors that bind to the sterol regulatory element DNA sequence TCACNCCAC. Mammalian SREBPs are encoded by the genes ''SREBF1'' and ''SREBF2''. SREBPs belong to the basic-helix-loop-helix leucine zipper class of transcription factors. Unactivated SREBPs are attached to the nuclear envelope and endoplasmic reticulum membranes. In cells with low levels of sterols, SREBPs are cleaved to a water-soluble N-terminal domain that is translocated to the nucleus. These activated SREBPs then bind to specific sterol regulatory element DNA sequences, thus upregulating the synthesis of enzymes involved in sterol biosynthesis. Sterols in turn inhibit the cleavage of SREBPs and therefore synthesis of additional sterols is reduced through a negative feed back loop. Isoforms Mammalian genomes have two separate SREBP genes ( and ): * SREBP-1 expression produces two different isoforms, SREBP-1a and -1c. These isoforms differ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insulin-induced Gene 1 Protein
Insulin induced gene 1, also known as INSIG1, is a protein which in humans is encoded by the ''INSIG1'' gene. INSIG1 is short for insulin-induced gene 1; it is located on chromosome 7 (7q36). This human gene encodes for a transmembrane protein of 277 amino acids with probably 6 transmembrane domains. It is localized in the endoplasmic reticulum (ER) and seems to be expressed in all tissues, especially in liver. This gene is called an insulin-induced gene because the molecule insulin can regulate it. Importantly, the protein encoded by this gene plays a critical role in regulating cholesterol concentrations in cells. Function #INSIG1 plays an important role in the SREBP-mediated regulation of cholesterol biosynthesis: by binding to the sterol-sensing domain of SCAP (SREBP cleavage activating protein) it makes the SCAP/SREBP complex stay longer in the ER, thus prohibiting SCAP from carrying activated SREBP to the golgi complex. This ultimately blocks SREBP from acting as a transcr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SREBP Cleavage-activating Protein
Sterol regulatory element-binding protein cleavage-activating protein, also known as SREBP cleavage-activating protein or SCAP is a protein that in humans is encoded by the ''SCAP'' gene. SCAP contains a sterol-sensing domain (SSD) and seven WD domains. In cholesterol-depleted cells, this protein binds to sterol regulatory element binding proteins (SREBPs) and mediates their transport from the ER to the Golgi apparatus. The SREBPs are then proteolytically cleaved and stimulate sterol biosynthesis. Function SCAP is a regulatory protein that is required for the proteolytic cleavage of the sterol regulatory element-binding protein (SREBP). SCAP is an integral membrane protein located in the endoplasmic reticulum (ER). One of the cytosolic regions of SCAP contains a hexapeptide amino acid sequence, MELADL, that functions to detect cellular cholesterol. When cholesterol is present, SCAP undergoes a conformational change that prevents it from activating SREBP and cholesterol synth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
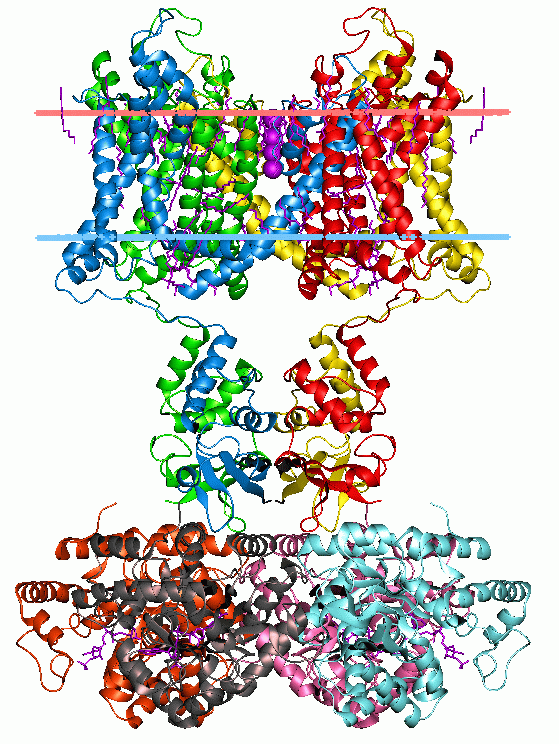
Membrane Protein
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane and can either penetrate the membrane (transmembrane) or associate with one or the other side of a membrane ( integral monotopic). Peripheral membrane proteins are transiently associated with the cell membrane. Membrane proteins are common, and medically important—about a third of all human proteins are membrane proteins, and these are targets for more than half of all drugs. Nonetheless, compared to other classes of proteins, determining membrane protein structures remains a challenge in large part due to the difficulty in establishing experimental conditions that can preserve the correct conformation of the protein in isolation from its native environment. Function Membrane proteins perform a variety of functions vital to the sur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |