|
Nucleotide Sugar
Nucleotide sugars are the activated forms of monosaccharides. Nucleotide sugars act as glycosyl donors in glycosylation reactions. Those reactions are catalyzed by a group of enzymes called glycosyltransferases. History The anabolism of oligosaccharides - and, hence, the role of nucleotide sugars - was not clear until the 1950s when Leloir and his coworkers found that the key enzymes in this process are the glycosyltransferases. These enzymes transfer a glycosyl group from a sugar nucleotide to an acceptor. Biological importance and energetics To act as glycosyl donors, those monosaccharides should exist in a highly energetic form. This occurs as a result of a reaction between nucleoside triphosphate (NTP) and glycosyl monophosphate (phosphate at anomeric carbon). The recent discovery of the reversibility of many glycosyltransferase-catalyzed reactions calls into question the designation of sugar nucleotides as 'activated' donors. Types There are nine sugar nucleotides in hum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monosaccharides
Monosaccharides (from Greek ''monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built. They are usually colorless, water-soluble, and crystalline solids. Contrary to their name (sugars), only some monosaccharides have a sweet taste. Most monosaccharides have the formula (though not all molecules with this formula are monosaccharides). Examples of monosaccharides include glucose (dextrose), fructose (levulose), and galactose. Monosaccharides are the building blocks of disaccharides (such as sucrose and lactose) and polysaccharides (such as cellulose and starch). The table sugar used in everyday vernacular is itself a disaccharide sucrose comprising one molecule of each of the two monosaccharides D-glucose and D-fructose. Each carbon atom that supports a hydroxyl group is chiral, except those at the end of the chain. This gives rise to a number of isomer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
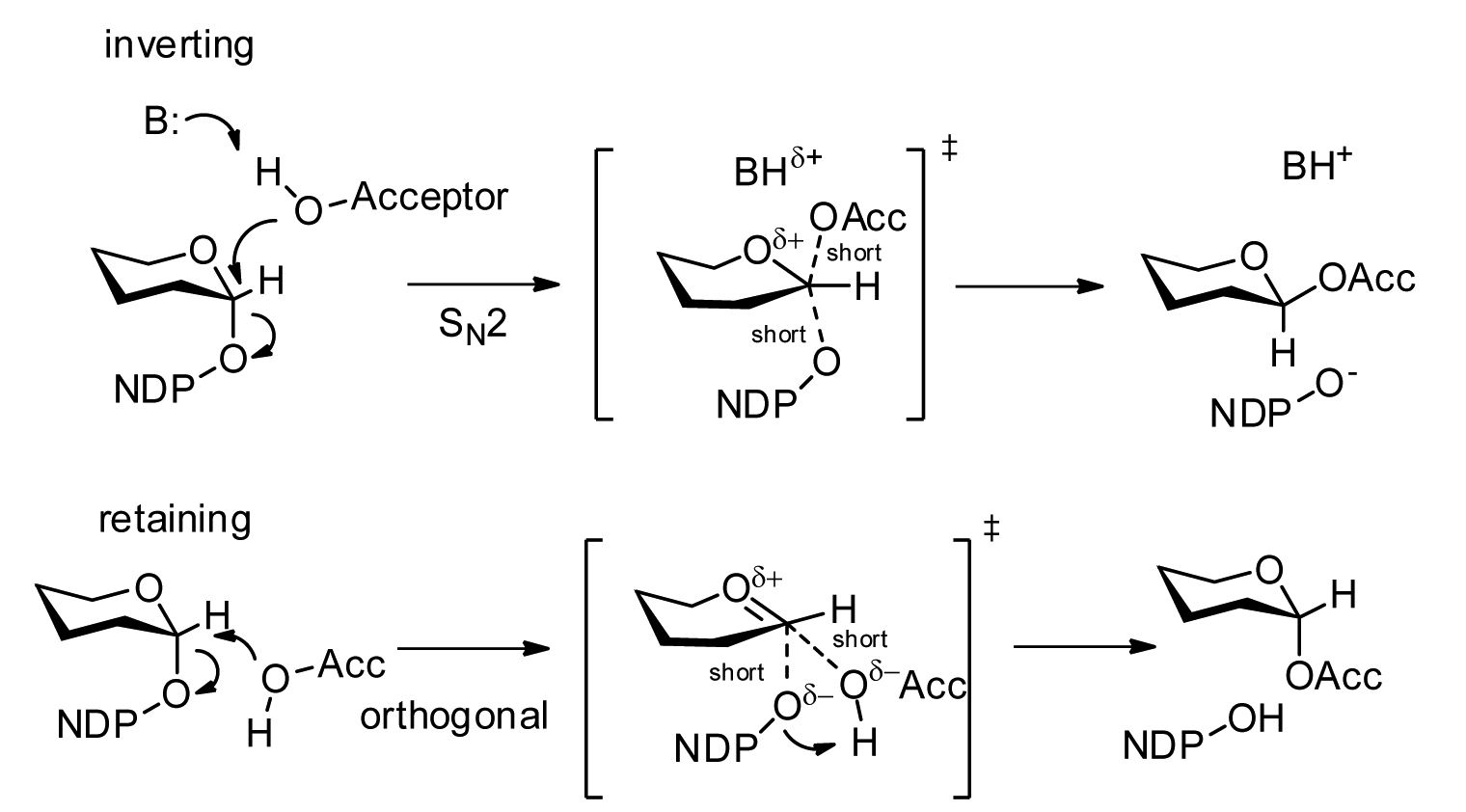
Glycosyltransferase
Glycosyltransferases (GTFs, Gtfs) are enzymes ( EC 2.4) that establish natural glycosidic linkages. They catalyze the transfer of saccharide moieties from an activated nucleotide sugar (also known as the "glycosyl donor") to a nucleophilic glycosyl acceptor molecule, the nucleophile of which can be oxygen- carbon-, nitrogen-, or sulfur-based. The result of glycosyl transfer can be a carbohydrate, glycoside, oligosaccharide, or a polysaccharide. Some glycosyltransferases catalyse transfer to inorganic phosphate or water. Glycosyl transfer can also occur to protein residues, usually to tyrosine, serine, or threonine to give O-linked glycoproteins, or to asparagine to give N-linked glycoproteins. Mannosyl groups may be transferred to tryptophan to generate C-mannosyl tryptophan, which is relatively abundant in eukaryotes. Transferases may also use lipids as an acceptor, forming glycolipids, and even use lipid-linked sugar phosphate donors, such as dolichol phosphates in eukaryotic o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycorandomization
Glycorandomization, is a drug discovery and drug development technology platform to enable the rapid diversification of bioactive small molecules, drug leads and/or approved drugs through the attachment of sugars. Initially developed as a facile method to manipulate carbohydrate substitutions of naturally occurring glycosides to afford the corresponding differentially glycosylated natural product libraries, glycorandomization applications have expanded to include both small molecules (drug leads and approved drugs) and even macromolecules (proteins). Also referred to as 'glycodiversification', glycorandomization has led to the discovery of new glycoside analogs which display improvements in potency, selectivity and/or ADME-Tox, ADMET as compared to the parent molecule. Classification The traditional method for attaching sugars to natural products, drugs or drug leads is by chemical glycosylation. This classical approach typically requires multiple protection/deprotection steps i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EamA
EamA (named after the O-acetyl-serine/cysteine export gene in '' E. coli'') is a protein domain found in a wide range of proteins including the ''Erwinia chrysanthemi'' PecM protein, which is involved in pectinase, cellulase and blue pigment regulation, the ''Salmonella typhimurium'' PagO protein (function unknown), and some members of the solute carrier family group 35 (SLC35) nucleoside-sugar transporters. Many members of this family have no known function and are predicted to be integral membrane proteins and many of the proteins contain two copies of the domain. Domain EamA was previously called DUF6 (domain unknown function) 6, and was one of the first DUF families to appear in Pfam. Maximum likelihood phylogenetic analysis indicates that this family contains four stable sub-families with high bootstrap values: SLC35C/E, SLC35F, SLC35G (acyl-malonyl condensing enzyme-like AMAC), and purine permeases. The EamA HMM domain organization shows the two domain structure of Ea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbohydrate Chemistry
Carbohydrate chemistry is a subdiscipline of chemistry primarily concerned with the detection, synthesis, structure, and function of carbohydrates. Due to the general structure of carbohydrates, their synthesis is often preoccupied with the selective formation of glycosidic linkages and the selective reaction of hydroxyl groups; as a result, it relies heavily on the use of protecting groups. Monosaccharides Individual saccharide residues are termed monosaccharides. Carbohydrate synthesis Carbohydrate synthesis is a sub-field of organic chemistry concerned specifically with the generation of natural and unnatural carbohydrate structures. This can include the synthesis of monosaccharide residues or structures containing more than one monosaccharide, known as oligosaccharides. Glycosidic bond formation * Chemical glycosylation * Fischer glycosidation * Glycosyl halide * Koenigs-Knorr reaction Protecting groups * Carbohydrate acetalisation * Trimethylsilyl * Benzyl Ether * para ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical synthesis (both semisynthesis and total synthesis) and have played a central role in the development of the field of organic chemistry by providing challenging synthetic targets. The term natural product has also been extended for commercial purposes to refer to cosmetics, dietary supplements, and foods produced from natural sources without added artificial ingredients. Within the field of organic chemistry, the definition of natural products is usually restricted to organic compounds isolated from natural sources that are produced by the pathways of primary or secondary metabolism. Within the field of medicinal chemistry, the definition is often further restricted to secondary metabolites. Secondary metabolites (or specialized metabolites ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmaceuticals
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate management. Drugs are classified in multiple ways. One of the key divisions is by level of control, which distinguishes prescription drugs (those that a pharmacist dispenses only on the order of a physician, physician assistant, or qualified nurse) from over-the-counter drugs (those that consumers can order for themselves). Another key distinction is between traditional small molecule drugs, usually derived from chemical synthesis, and biopharmaceuticals, which include recombinant proteins, vaccines, blood products used therapeutically (such as IVIG), gene therapy, monoclonal antibodies and cell therapy (for instance, stem cell therapies). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosyltransferases
Glycosyltransferases (GTFs, Gtfs) are enzymes ( EC 2.4) that establish natural glycosidic linkages. They catalyze the transfer of saccharide moieties from an activated nucleotide sugar (also known as the "glycosyl donor") to a nucleophilic glycosyl acceptor molecule, the nucleophile of which can be oxygen- carbon-, nitrogen-, or sulfur-based. The result of glycosyl transfer can be a carbohydrate, glycoside, oligosaccharide, or a polysaccharide. Some glycosyltransferases catalyse transfer to inorganic phosphate or water. Glycosyl transfer can also occur to protein residues, usually to tyrosine, serine, or threonine to give O-linked glycoproteins, or to asparagine to give N-linked glycoproteins. Mannosyl groups may be transferred to tryptophan to generate C-mannosyl tryptophan, which is relatively abundant in eukaryotes. Transferases may also use lipids as an acceptor, forming glycolipids, and even use lipid-linked sugar phosphate donors, such as dolichol phosphates in eukaryotic o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycorandomization
Glycorandomization, is a drug discovery and drug development technology platform to enable the rapid diversification of bioactive small molecules, drug leads and/or approved drugs through the attachment of sugars. Initially developed as a facile method to manipulate carbohydrate substitutions of naturally occurring glycosides to afford the corresponding differentially glycosylated natural product libraries, glycorandomization applications have expanded to include both small molecules (drug leads and approved drugs) and even macromolecules (proteins). Also referred to as 'glycodiversification', glycorandomization has led to the discovery of new glycoside analogs which display improvements in potency, selectivity and/or ADME-Tox, ADMET as compared to the parent molecule. Classification The traditional method for attaching sugars to natural products, drugs or drug leads is by chemical glycosylation. This classical approach typically requires multiple protection/deprotection steps i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GDP-Man
Guanosine diphosphate mannose or GDP-mannose is a nucleotide sugar that is a substrate for glycosyltransferase reactions in metabolism. This compound is a substrate for enzymes called mannosyltransferases. Known as donor of activated mannose in all glycolytic reactions, GDP-mannose is essential in eukaryotes. Biosynthesis GDP-mannose is produced from GTP and mannose-6-phosphate by the enzyme mannose-1-phosphate guanylyltransferase. One of the enzymes from the family of nucleootidyl-transferases, GDP-Mannose Pyrophosphorylase (GDP-MP) is an pervasive enzyme found in bacteria, fungi, plants, and animals. References See also * Nucleoside * Nucleotide * Guanosine * Guanosine diphosphate Guanosine diphosphate, abbreviated GDP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside guanosine. GDP consists of a pyrophosphate group, a pentose sugar ribose, and the nucleobase guanine. GDP is the product ... Nucleotides Coenzymes {{bioch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |