|
Magnesite
Magnesite is a mineral with the chemical formula ( magnesium carbonate). Iron, manganese, cobalt, and nickel may occur as admixtures, but only in small amounts. Occurrence Magnesite occurs as veins in and an alteration product of ultramafic rocks, serpentinite and other magnesium rich rock types in both contact and regional metamorphic terrains. These magnesites are often cryptocrystalline and contain silica in the form of opal or chert. Magnesite is also present within the regolith above ultramafic rocks as a secondary carbonate within soil and subsoil, where it is deposited as a consequence of dissolution of magnesium-bearing minerals by carbon dioxide in groundwaters. Isotopic structure: clumped isotope The recent advancement in the field of stable isotope geochemistry is the study of isotopic structure of minerals and molecules. This requires study of molecules with high resolutions looking at bonding scenario (how heavy isotopes are bonded to each other)- leading ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Carbonate
Magnesium carbonate, (archaic name magnesia alba), is an inorganic salt that is a colourless or white solid. Several hydrated and basic forms of magnesium carbonate also exist as minerals. Forms The most common magnesium carbonate forms are the anhydrous salt called magnesite (), and the di, tri, and pentahydrates known as barringtonite (), nesquehonite (), and lansfordite (), respectively. Some basic forms such as artinite (), hydromagnesite (), and dypingite () also occur as minerals. All of those minerals are colouress or white. Magnesite consists of colourless or white trigonal crystals. The anhydrous salt is practically insoluble in water, acetone, and ammonia. All forms of magnesium carbonate react with acids. Magnesite crystallizes in the calcite structure wherein is surrounded by six oxygen atoms. The dihydrate has a triclinic structure, while the trihydrate has a monoclinic structure. References to "light" and "heavy" magnesium carbonates actually refer t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate Mineral
Carbonate minerals are those minerals containing the carbonate ion, . Carbonate divisions Anhydrous carbonates *Calcite group: trigonal ** Calcite CaCO3 ** Gaspéite (Ni,Mg,Fe2+)CO3 **Magnesite MgCO3 ** Otavite CdCO3 ** Rhodochrosite MnCO3 ** Siderite FeCO3 ** Smithsonite ZnCO3 **Spherocobaltite CoCO3 *Aragonite group: orthorhombic ** Aragonite CaCO3 ** Cerussite PbCO3 ** Strontianite SrCO3 ** Witherite BaCO3 ** Rutherfordine UO2CO3 ** Natrite Na2CO3 Anhydrous carbonates with compound formulas *Dolomite group: trigonal ** Ankerite CaFe(CO3)2 ** Dolomite CaMg(CO3)2 ** Huntite Mg3Ca(CO3)4 ** Minrecordite CaZn(CO3)2 ** Barytocalcite BaCa(CO3)2 Carbonates with hydroxyl or halogen *Carbonate with hydroxide: monoclinic **Azurite Cu3(CO3)2(OH)2 ** Hydrocerussite Pb3(CO3)2(OH)2 ** Malachite Cu2CO3(OH)2 ** Rosasite (Cu,Zn)2CO3(OH)2 ** Phosgenite Pb2(CO3)Cl2 ** Hydrozincite Zn5(CO3)2(OH)6 ** Aurichalcite (Zn,Cu)5(CO3)2(OH)6 Hydrated carbonates * Hydromagnesite Mg5(CO3)4(OH)2.4H2O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dolomite (mineral)
Dolomite () is an anhydrous carbonate mineral composed of calcium magnesium carbonate, ideally The term is also used for a sedimentary carbonate rock composed mostly of the mineral dolomite. An alternative name sometimes used for the dolomitic rock type is dolostone. History As stated by Nicolas-Théodore de Saussure the mineral dolomite was probably first described by Carl Linnaeus in 1768. In 1791, it was described as a rock by the French naturalist and geologist Déodat Gratet de Dolomieu (1750–1801), first in buildings of the old city of Rome, and later as samples collected in the mountains now known as the Dolomite Alps of northern Italy. Nicolas-Théodore de Saussure first named the mineral (after Dolomieu) in March 1792. Properties The mineral dolomite crystallizes in the trigonal-rhombohedral system. It forms white, tan, gray, or pink crystals. Dolomite is a double carbonate, having an alternating structural arrangement of calcium and magnesium ions. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultramafic Rocks
Ultramafic rocks (also referred to as ultrabasic rocks, although the terms are not wholly equivalent) are igneous and meta-igneous rocks with a very low silica content (less than 45%), generally >18% MgO, high FeO, low potassium, and are composed of usually greater than 90% mafic minerals (dark colored, high magnesium and iron content). The Earth's mantle is composed of ultramafic rocks. Ultrabasic is a more inclusive term that includes igneous rocks with low silica content that may not be extremely enriched in Fe and Mg, such as carbonatites and ultrapotassic igneous rocks. Intrusive ultramafic rocks Intrusive ultramafic rocks are often found in large, layered ultramafic intrusions where differentiated rock types often occur in layers. Such cumulate rock types do not represent the chemistry of the magma from which they crystallized. The ultramafic intrusives include the dunites, peridotites and pyroxenites. Other rare varieties include troctolite which has a greater per ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Siderite
Siderite is a mineral composed of iron(II) carbonate (FeCO3). It takes its name from the Greek word σίδηρος ''sideros,'' "iron". It is a valuable iron mineral, since it is 48% iron and contains no sulfur or phosphorus. Zinc, magnesium and manganese commonly substitute for the iron resulting in the siderite- smithsonite, siderite-magnesite and siderite-rhodochrosite solid solution series. Siderite has Mohs hardness of 3.75-4.25, a specific gravity of 3.96, a white streak and a vitreous lustre or pearly luster. Siderite is antiferromagnetic below its Néel temperature of 37 K which can assist in its identification. It crystallizes in the trigonal crystal system, and are rhombohedral in shape, typically with curved and striated faces. It also occurs in masses. Color ranges from yellow to dark brown or black, the latter being due to the presence of manganese. Siderite is commonly found in hydrothermal veins, and is associated with barite, fluorite, galena, and oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcite
Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on scratch hardness comparison. Large calcite crystals are used in optical equipment, and limestone composed mostly of calcite has numerous uses. Other polymorphs of calcium carbonate are the minerals aragonite and vaterite. Aragonite will change to calcite over timescales of days or less at temperatures exceeding 300 °C, and vaterite is even less stable. Etymology Calcite is derived from the German ''Calcit'', a term from the 19th century that came from the Latin word for lime, ''calx'' (genitive calcis) with the suffix "-ite" used to name minerals. It is thus etymologically related to chalk. When applied by archaeologists and stone trade professionals, the term alabaster is used not just as in geology and mineralogy, where it is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clumped Isotopes
Clumped isotopes are heavy isotopes that are bonded to other heavy isotopes. The relative abundance of clumped isotopes (and multiply-substituted isotopologues) in molecules such as methane, nitrous oxide, and carbonate is an area of active investigation. The carbonate clumped-isotope thermometer, or "13C–18O order/disorder carbonate thermometer", is a new approach for paleoclimate reconstruction, based on the temperature dependence of the clumping of 13C and 18O into bonds within the carbonate mineral lattice. This approach has the advantage that the 18O ratio in water is not necessary (different from the δ18O approach), but for precise paleotemperature estimation, it also needs very large and uncontaminated samples, long analytical runs, and extensive replication. Commonly used sample sources for paleoclimatological work include corals, otoliths, gastropods, tufa, bivalves, and foraminifera. Results are usually expressed as Δ47 (said as "cap 47"), which is the devi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
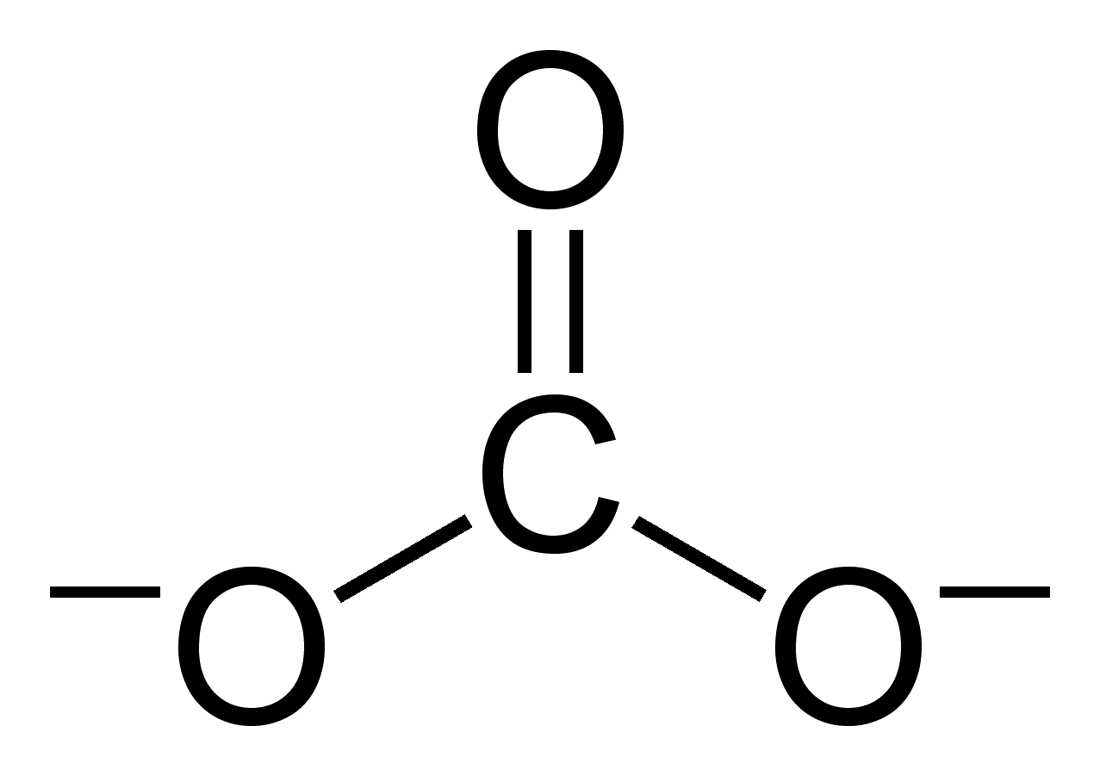
Carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2. The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverageseither by the addition of carbon dioxide gas under pressure or by dissolving carbonate or bicarbonate salts into the water. In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock. The most common are calcite or calcium carbonate, CaCO3, the chief constituent of limestone (as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |