|
Isometheptene
Isometheptene (usually as isometheptene mucate) is a sympathomimetic amine sometimes used in the treatment of migraines and tension headaches due to its vasoconstricting properties; that is, it causes constriction (narrowing) of blood vessels (arteries and veins). Along with paracetamol and dichloralphenazone, it is one of the constituents of Amidrine. Chemistry Isometheptene is a monounsaturated aliphatic secondary amine. Mechanism of action Isometheptene's vasoconstricting properties arise through activation of the sympathetic nervous system via epinephrine and norepinephrine. These compounds elicit smooth muscle activation leading to vasoconstriction by interacting with cell surface adrenergic receptors. See also * Heptaminol * Methylhexanamine * Tuaminoheptane Tuaminoheptane (, ; brand names Heptin, Heptadrine, Tuamine; also known as tuamine and 2-aminoheptane) is a sympathomimetic agent and vasoconstrictor which was formerly used as a nasal decongestant. It has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylhexanamine
Methylhexanamine (also known as methylhexamine, 1,3-dimethylamylamine, 1,3-DMAA, dimethylamylamine, and DMAA; trade names Forthane and Geranamine) is an indirect sympathomimetic drug invented and developed by Eli Lilly and Company and marketed as an inhaled nasal decongestant from 1948 until it was voluntarily withdrawn from the market in the 1970s. Since 2006 methylhexanamine has been sold extensively under many names as a stimulant or energy-boosting dietary supplement under the claim that it is similar to certain compounds found in geraniums, but its safety has been questioned as a number of adverse events and at least five deaths have been associated with methylhexanamine-containing supplements. It is banned by many sports authorities and governmental agencies. Despite multiple warning letters from the FDA, as of 2019, the stimulant remains available in sports and weight loss supplements. History In April 1944, Eli Lilly and Company introduced methylhexanamine under the bra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichloralphenazone
Dichloralphenazone is a 1:2 mixture of antipyrine with chloral hydrate. In combination with paracetamol and isometheptene, it is the active ingredient of medications for migraine and tension headaches, including Epidrin and Midrin. Performance impairments are common with this drug and caution is advised, for example when driving motor vehicles. Additional uses of dichloralphenazone include sedation for the treatment of short-term insomnia, although there are probably better drug choices for the treatment of insomnia. See also * Chloral betaine Chloral betaine (USAN, BAN) (brand names Beta-Chlor, Somilan), also known as cloral betaine (INN), is a sedative- hypnotic drug. It was introduced by Mead Johnson in the United States in 1963. It is a betaine complex with chloral hydrate, whi ... References External links * Analgesics Hypnotics Sedatives Combination drugs GABAA receptor positive allosteric modulators {{sedative-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amidrine
Amidrine, Midrin, Nodolor, Duradrin, IDA, Migquin, Migrin-A, Migrazone or Epidrine is a combination drug consisting of paracetamol, dichloralphenazone and isometheptene used to treat migraines and severe, refractory headaches. Components * Paracetamol, also known as acetaminophen, is a common over-the-counter pain reliever and fever reducer. * Dichloralphenazone is a prodrug of two pharmacologically distinct agents: the sedative agent, chloral hydrate, as well as antipyrine, a non-steroidal anti-inflammatory drug that works to decrease inflammation. * Isometheptene is a sympathomimetic drug that works by inducing vasoconstriction, causing constriction of cerebral blood vessels and reducing migraine symptoms. Availability Midrin was discontinued by Caraco Pharmaceuticals as of 2009, after an FDA seizure of 33 drugs manufactured by Caraco Pharmaceuticals due to cGMP (Current Good Manufacturing Practices) violations. Generic forms of Amidrine were also discontinued due to loss of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mucic Acid
Mucic acid, C6H10O8 or HOOC-(CHOH)4-COOH (also known as galactaric or meso-galactaric acid) is an aldaric acid obtained by nitric acid oxidation of galactose or galactose-containing compounds such as lactose, dulcite, quercite, and most varieties of gum. Properties Mucic acid forms a crystalline powder, which melts at 210–230 °C. It is insoluble in alcohol, and nearly insoluble in cold water. Due to the symmetry in the molecule, it is optically inactive even though it has chiral carbon atoms (i.e., it is a meso compound). Reactions When heated with pyridine to 140 °C, it is converted into allomucic acid. When digested with fuming hydrochloric acid for some time it is converted into αα′ furfural dicarboxylic acid while on heating with barium sulfide it is transformed into a thiophene carboxylic acid. The ammonium salt yields on dry distillation carbon dioxide, ammonia, pyrrol and other substances. The acid when fused with caustic alkalis yields oxalic acid. W ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
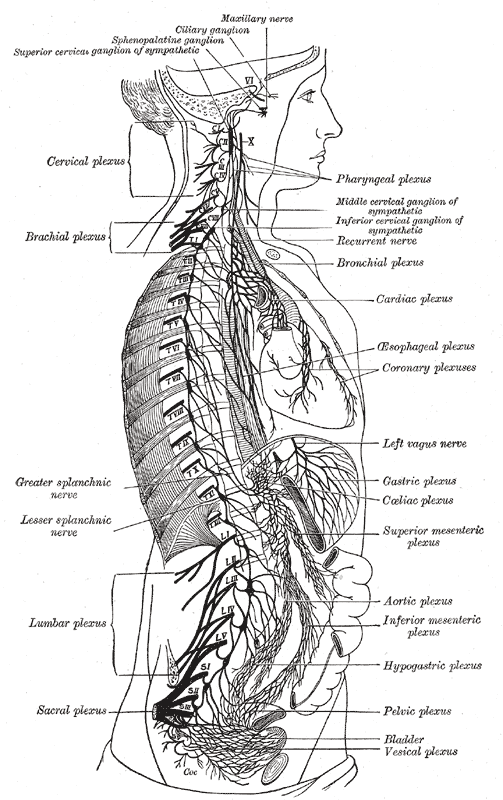
Sympathetic Nervous System
The sympathetic nervous system (SNS) is one of the three divisions of the autonomic nervous system, the others being the parasympathetic nervous system and the enteric nervous system. The enteric nervous system is sometimes considered part of the autonomic nervous system, and sometimes considered an independent system. The autonomic nervous system functions to regulate the body's unconscious actions. The sympathetic nervous system's primary process is to stimulate the body's fight or flight response. It is, however, constantly active at a basic level to maintain homeostasis. The sympathetic nervous system is described as being antagonistic to the parasympathetic nervous system which stimulates the body to "feed and breed" and to (then) "rest-and-digest". Structure There are two kinds of neurons involved in the transmission of any signal through the sympathetic system: pre-ganglionic and post-ganglionic. The shorter preganglionic neurons originate in the thoracolumbar division o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene Derivatives
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tuaminoheptane
Tuaminoheptane (, ; brand names Heptin, Heptadrine, Tuamine; also known as tuamine and 2-aminoheptane) is a sympathomimetic agent and vasoconstrictor which was formerly used as a nasal decongestant. It has also been used as a stimulant. Tuaminoheptane has been found to act as a reuptake inhibitor and releasing agent of norepinephrine, which may underlie its decongestant and stimulant effects. It is an alkylamine. The chemical structure of the drug differs from that of other norepinephrine releasing agents, such as the phenethylamines, which, in contrast to tuaminoheptane, have an aromatic ring in their structure. Tuaminoheptane is also a skin irritant and can cause contact dermatitis via inhibition of volume-regulated anion channels, which limits its usefulness as a decongestant. Tuaminoheptane is on the 2011 list of prohibited substances published by the World Anti-Doping Agency. See also * 1,3-Dimethylbutylamine * Heptaminol * Isometheptene * Methylhexanamine Methylhexa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heptaminol
Heptaminol is an amino alcohol which is classified as a cardiac stimulant (positive inotropic action). It also increases coronary blood flow along with mild peripheral vasoconstriction. It is sometimes used in the treatment of low blood pressure, particularly orthostatic hypotension as it is a potent positive inotrope (improving cardiac contraction). Use in doping Heptaminol is classified by the World Anti-Doping Agency as a doping substance. In 2008, the cyclist Dmitriy Fofonov tested positive for heptaminol at the Tour de France. In June 2010, the swimmer Frédérick Bousquet tested positive. In 2013, the cyclist Sylvain Georges tested positive at the Giro d'Italia. In 2014, baseball player Joel Piniero tested positive as well as St. Louis Cardinals minor league baseball player Yeison Medina. On March 22, 2019, Cycling South Africa reported that Ricardo Broxham has been sanctioned for an anti-doping rule violation of Articles 2.1 and 2.2 of the UCI Anti-Doping Rules after an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adrenergic Receptor
The adrenergic receptors or adrenoceptors are a class of G protein-coupled receptors that are targets of many catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) produced by the body, but also many medications like beta blockers, beta-2 (β2) agonists and alpha-2 (α2) agonists, which are used to treat high blood pressure and asthma, for example. Many cells have these receptors, and the binding of a catecholamine to the receptor will generally stimulate the sympathetic nervous system (SNS). The SNS is responsible for the fight-or-flight response, which is triggered by experiences such as exercise or fear-causing situations. This response dilates pupils, increases heart rate, mobilizes energy, and diverts blood flow from non-essential organs to skeletal muscle. These effects together tend to increase physical performance momentarily. History By the turn of the 19th century, it was agreed that the stimulation of sympathetic nerves could cause different ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
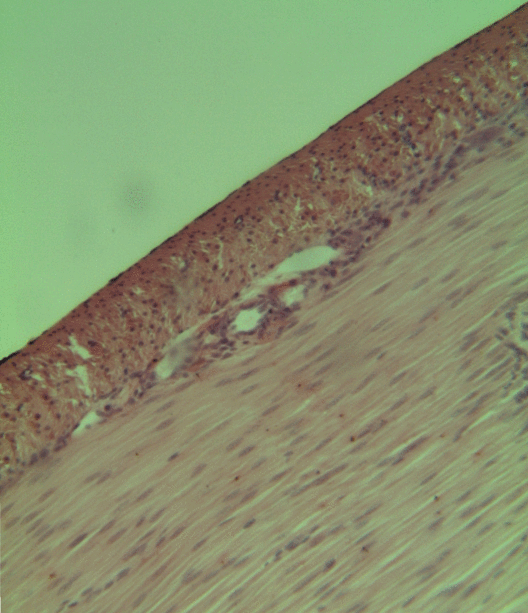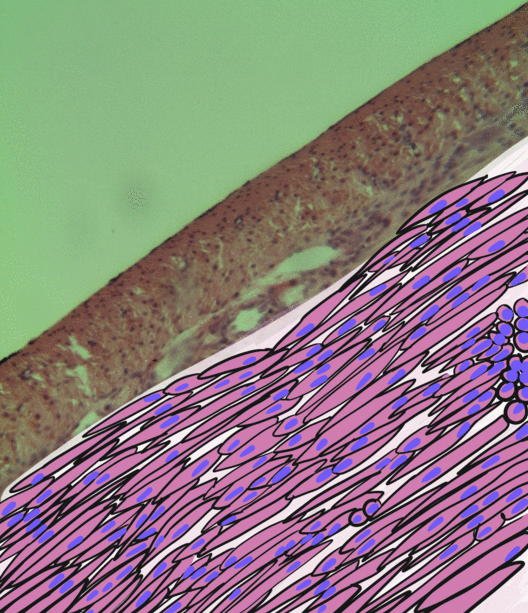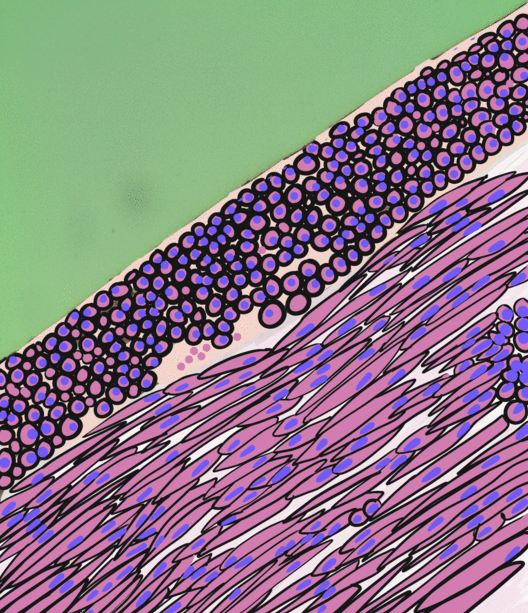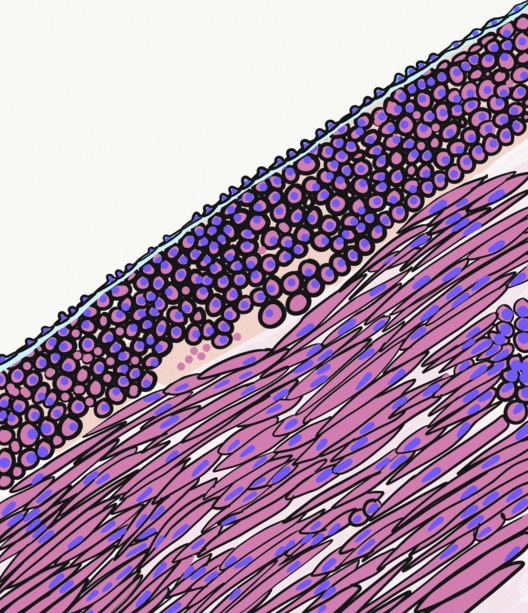
Smooth Muscle Tissue
Smooth muscle is an involuntary non-striated muscle, so-called because it has no sarcomeres and therefore no striations (''bands'' or ''stripes''). It is divided into two subgroups, single-unit and multiunit smooth muscle. Within single-unit muscle, the whole bundle or sheet of smooth muscle cells contracts as a syncytium. Smooth muscle is found in the walls of hollow organs, including the stomach, intestines, bladder and uterus; in the walls of passageways, such as blood, and lymph vessels, and in the tracts of the respiratory, urinary, and reproductive systems. In the eyes, the ciliary muscles, a type of smooth muscle, dilate and contract the iris and alter the shape of the lens. In the skin, smooth muscle cells such as those of the arrector pili cause hair to stand erect in response to cold temperature or fear. Structure Gross anatomy Smooth muscle is grouped into two types: single-unit smooth muscle, also known as visceral smooth muscle, and multiunit smooth muscle. M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Norepinephrine
Norepinephrine (NE), also called noradrenaline (NA) or noradrenalin, is an organic chemical in the catecholamine family that functions in the brain and body as both a hormone and neurotransmitter. The name "noradrenaline" (from Latin '' ad'', "near", and '' ren'', "kidney") is more commonly used in the United Kingdom, whereas "norepinephrine" (from Ancient Greek ἐπῐ́ (''epí''), "upon", and νεφρός (''nephrós''), "kidney") is usually preferred in the United States. "Norepinephrine" is also the international nonproprietary name given to the drug. Regardless of which name is used for the substance itself, parts of the body that produce or are affected by it are referred to as noradrenergic. The general function of norepinephrine is to mobilize the brain and body for action. Norepinephrine release is lowest during sleep, rises during wakefulness, and reaches much higher levels during situations of stress or danger, in the so-called fight-or-flight response. In the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epinephrine
Adrenaline, also known as epinephrine, is a hormone and medication which is involved in regulating visceral functions (e.g., respiration). It appears as a white microcrystalline granule. Adrenaline is normally produced by the adrenal glands and by a small number of neurons in the medulla oblongata. It plays an essential role in the fight-or-flight response by increasing blood flow to muscles, heart output by acting on the SA node, pupil dilation response, and blood sugar level. It does this by binding to alpha and beta receptors. It is found in many animals, including humans, and some single-celled organisms. It has also been isolated from the plant ''Scoparia dulcis'' found in Northern Vietnam. Medical uses As a medication, it is used to treat several conditions, including allergic reaction anaphylaxis, cardiac arrest, and superficial bleeding. Inhaled adrenaline may be used to improve the symptoms of croup. It may also be used for asthma when other treatments are not e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |