|
Hemidesmosome
Hemidesmosomes are very small stud-like structures found in keratinocytes of the epidermis of skin that attach to the extracellular matrix. They are similar in form to desmosomes when visualized by electron microscopy, however, desmosomes attach to adjacent cells. Hemidesmosomes are also comparable to focal adhesions, as they both attach cells to the extracellular matrix. Instead of desmogleins and desmocollins in the extracellular space, hemidesmosomes utilize integrins. Hemidesmosomes are found in epithelial cells connecting the basal epithelial cells to the lamina lucida, which is part of the basal lamina. Hemidesmosomes are also involved in signaling pathways, such as keratinocyte migration or carcinoma cell intrusion. Structure Hemidesmosomes can be categorized into two types based on their protein constituents. Type 1 hemidesmosomes are found in stratified and pseudo-stratified epithelium. Type 1 hemidesmosomes have five main elements: integrin α6 β4, plectin in its isof ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Integrin Alpha 6
Integrin alpha-6 is a protein that in humans is encoded by the ''ITGA6'' gene. Function The ITGA6 protein product is the integrin alpha chain alpha 6. Integrins are integral cell-surface proteins composed of an alpha chain and a beta chain. A given chain may combine with multiple partners resulting in different integrins. For example, alpha 6 may combine with beta 4 in the integrin referred to as TSP180, or with beta 1 in the integrin VLA-6. Integrins are known to participate in cell adhesion as well as cell-surface mediated signalling. Two transcript variants encoding different isoforms have been found for this gene. Specific loss of this integrin chain in the intestinal epithelium, and thus of their hemidesmosomes, induces long-standing colitis and infiltrating adenocarcinomas. Interactions ITGA6 has been shown to interact with TSPAN4 and GIPC1. See also * Cluster of differentiation * Integrin Integrins are transmembrane receptors that facilitate cell-cell and cell-ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extracellular Matrix
In biology, the extracellular matrix (ECM), also called intercellular matrix, is a three-dimensional network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM. The animal extracellular matrix includes the interstitial matrix and the basement membrane. Interstitial matrix is present between various animal cells (i.e., in the intercellular spaces). Gels of polysaccharides and fibrous proteins fill the Interstitial fluid, interstitial space and act as a compression buffer against the stress placed on the ECM. Basement membranes are sheet-like depositions of ECM on which various epithelial cells rest ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Desmosome
A desmosome (; "binding body"), also known as a macula adherens (plural: maculae adherentes) (Latin for ''adhering spot''), is a cell structure specialized for cell-to-cell adhesion. A type of junctional complex, they are localized spot-like adhesions randomly arranged on the lateral sides of plasma membranes. Desmosomes are one of the stronger cell-to-cell adhesion types and are found in tissue that experience intense mechanical stress, such as cardiac muscle tissue, bladder tissue, gastrointestinal mucosa, and epithelia. Structure Desmosomes are composed of desmosome-intermediate filament complexes (DIFC), which is a network of cadherin proteins, linker proteins and intermediate filaments. The DIFCs can be broken into three regions: the extracellular core region, or desmoglea, the outer dense plaque, or ODP, and the inner dense plaque, or IDP. The extracellular core region, approximately 34 nm in length, contains desmoglein and desmocollin, which are in the cadherin famil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Integrin Beta 4
Integrin, beta 4 (ITGB4) also known as CD104 (Cluster of Differentiation 104), is a human gene. Function Integrins are heterodimers composed of alpha and beta subunits, that are noncovalently associated transmembrane glycoprotein receptors. Different combinations of alpha and beta polypeptides form complexes that vary in their ligand-binding specificities. Integrins mediate cell-matrix or cell-cell adhesion, and transduced signals that regulate gene expression and cell growth. This gene encodes the integrin beta 4 subunit, a receptor for the laminins. This subunit tends to associate with alpha 6 subunit and is likely to play a pivotal role in the biology of invasive carcinoma. Mutations in this gene are associated with epidermolysis bullosa with pyloric atresia. Multiple alternatively spliced transcript variants encoding distinct isoforms have been found for this gene. Interactions ITGB4 has been shown to interact with Collagen, type XVII, alpha 1, EIF6 and Erbin. See al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
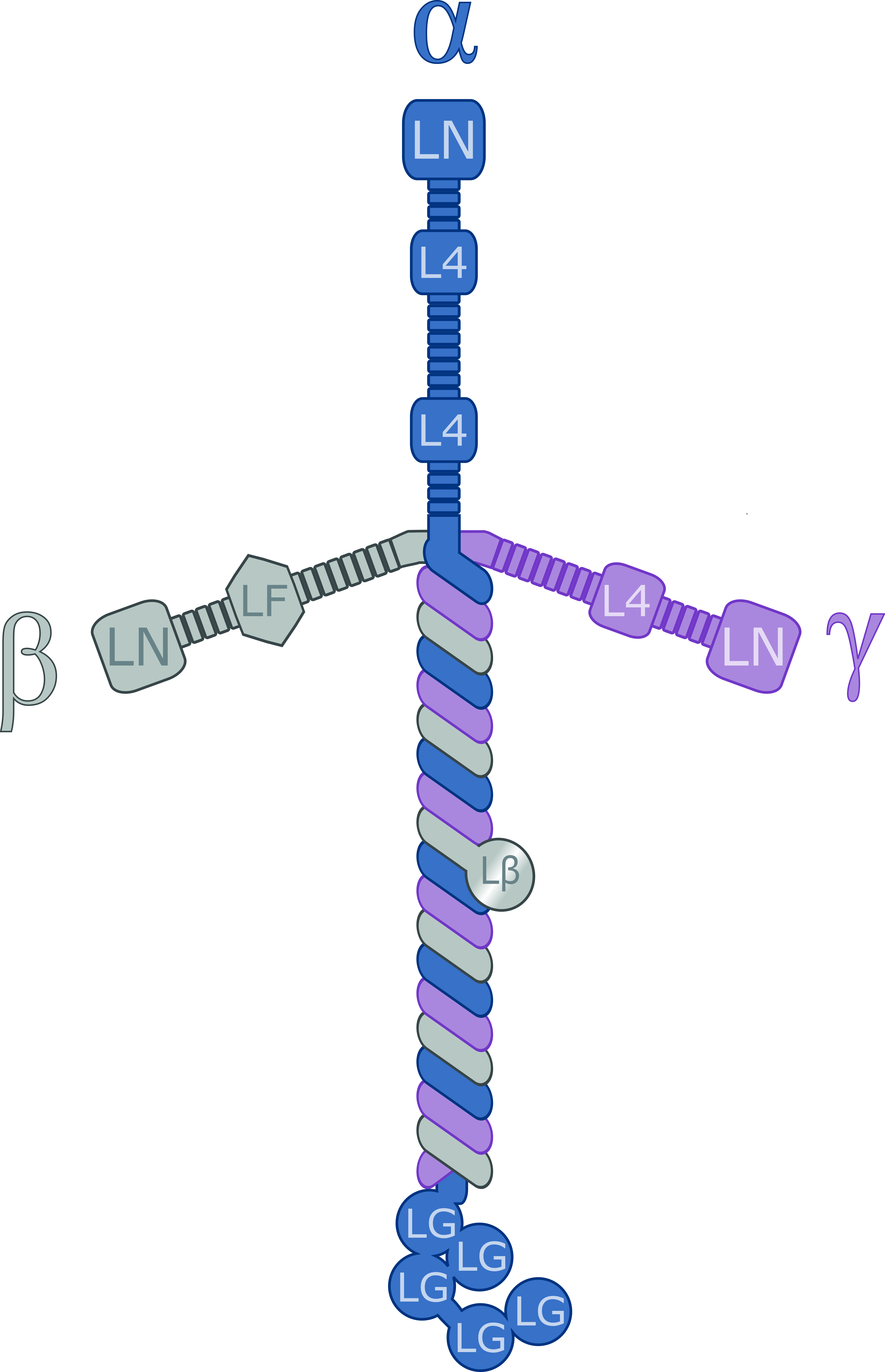
Laminin
Laminins are a family of glycoproteins of the extracellular matrix of all animals. They are major components of the basal lamina (one of the layers of the basement membrane), the protein network foundation for most cells and organs. The laminins are an important and biologically active part of the basal lamina, influencing cell differentiation, migration, and adhesion. Laminins are heterotrimeric proteins with a high molecular mass (~400 to ~900 kDa). They contain three different chains (α, β and γ) encoded by five, four, and three paralogous genes in humans, respectively. The laminin molecules are named according to their chain composition. Thus, laminin-511 contains α5, β1, and γ1 chains. Fourteen other chain combinations have been identified ''in vivo''. The trimeric proteins intersect to form a cross-like structure that can bind to other cell membrane and extracellular matrix molecules. The three shorter arms are particularly good at binding to other laminin molecules, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraspanin
Tetraspanins are a family of membrane proteins found in all multicellular eukaryotes. Tetraspanins, also referred to as the transmembrane 4 superfamily (TM4SF) proteins, have four transmembrane alpha-helices and two extracellular domains, one short (called the small extracellular domain or loop, SED/SEL or EC1) and one longer, typically 100 amino acid residues (the large extracellular domain/loop, LED/LEL or EC2). Although several protein families have four transmembrane alpha-helices, tetraspanins are defined by conserved amino acid sequences including four or more cysteine residues in the EC2 domain, with two in a highly conserved 'CCG' motif. Tetraspanins are often thought to act as scaffolding proteins, anchoring multiple proteins to one area of the cell membrane. Tetraspanins are highly conserved between species. Some tetraspanins can have ''N''-linked glycosylations on the long extracellular loop (LEL, EC2) and palmitoylations at a CXXC motif in their transmembrane region ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CD151
CD151 molecule (Raph blood group), also known as CD151 (Cluster of Differentiation 151), is a human gene. Function The protein encoded by this gene is a member of the transmembrane 4 superfamily, also known as the tetraspanin family. Most of these members are cell-surface proteins that are characterized by the presence of four hydrophobic domains. The proteins mediate signal transduction events that play a role in the regulation of cell development, activation, growth and motility. This encoded protein is a cell surface glycoprotein that is known to complex with integrins and other transmembrane 4 superfamily proteins. It is involved in cellular processes including cell adhesion and may regulate integrin trafficking and/or function. This protein enhances cell motility, invasion and metastasis of cancer cells. Multiple alternatively spliced transcript variants that encode the same protein have been described for this gene. Abnormalities in CD151 have been implicated in a form of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BPAG1
Dystonin (DST), also known as bullous pemphigoid antigen 1 (BPAG1), isoforms 1/2/3/4/5/8, is a protein that in humans is encoded by the ''DST'' gene. This gene encodes a member of the plakin protein family of adhesion junction plaque proteins. Multiple alternatively spliced transcript variants encoding distinct isoforms have been found for this gene, but the full-length nature of some variants has not been defined. It has been known that some isoforms are expressed in neural and muscle tissue, anchoring neural intermediate filaments to the actin cytoskeleton, and some isoforms are expressed in epithelial tissue, anchoring keratin-containing intermediate filaments to hemidesmosomes. Consistent with the expression, mice defective for this gene show skin blistering and neurodegeneration. Interactions Dystonin has been shown to interact with collagen, type XVII, alpha 1, DCTN1, MAP1B and erbin. Loss of function in neurological disease Several ''Dst'' mutant mouse lines have be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intermediate Filament
Intermediate filaments (IFs) are cytoskeletal structural components found in the cells of vertebrates, and many invertebrates. Homologues of the IF protein have been noted in an invertebrate, the cephalochordate ''Branchiostoma''. Intermediate filaments are composed of a family of related proteins sharing common structural and sequence features. Initially designated 'intermediate' because their average diameter (10 nm) is between those of narrower microfilaments (actin) and wider myosin filaments found in muscle cells, the diameter of intermediate filaments is now commonly compared to actin microfilaments (7 nm) and microtubules (25 nm). Animal intermediate filaments are subcategorized into six types based on similarities in amino acid sequence and protein structure. Most types are cytoplasmic, but one type, Type V is a nuclear lamin. Unlike microtubules, IF distribution in cells show no good correlation with the distribution of either mitochondria or endopla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
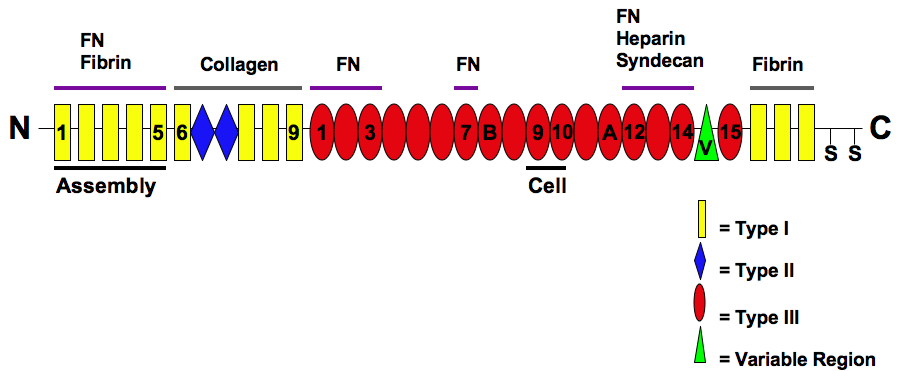
Fibronectin
Fibronectin is a high- molecular weight (~500-~600 kDa) glycoprotein of the extracellular matrix that binds to membrane-spanning receptor proteins called integrins. Fibronectin also binds to other extracellular matrix proteins such as collagen, fibrin, and heparan sulfate proteoglycans (e.g. syndecans). Fibronectin exists as a protein dimer, consisting of two nearly identical monomers linked by a pair of disulfide bonds. The fibronectin protein is produced from a single gene, but alternative splicing of its pre-mRNA leads to the creation of several isoforms. Two types of fibronectin are present in vertebrates: * soluble plasma fibronectin (formerly called "cold-insoluble globulin", or CIg) is a major protein component of blood plasma (300 μg/ml) and is produced in the liver by hepatocytes. * insoluble cellular fibronectin is a major component of the extracellular matrix. It is secreted by various cells, primarily fibroblasts, as a soluble protein dimer and is then ass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Keratin
Keratin () is one of a family of structural fibrous proteins also known as ''scleroproteins''. Alpha-keratin (α-keratin) is a type of keratin found in vertebrates. It is the key structural material making up scales, hair, nails, feathers, horns, claws, hooves, and the outer layer of skin among vertebrates. Keratin also protects epithelial cells from damage or stress. Keratin is extremely insoluble in water and organic solvents. Keratin monomers assemble into bundles to form intermediate filaments, which are tough and form strong unmineralized epidermal appendages found in reptiles, birds, amphibians, and mammals. Excessive keratinization participate in fortification of certain tissues such as in horns of cattle and rhinos, and armadillos' osteoderm. The only other biological matter known to approximate the toughness of keratinized tissue is chitin. Keratin comes in two types, the primitive, softer forms found in all vertebrates and harder, derived forms found only amon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |