|
Glycosylation
Glycosylation is the reaction in which a carbohydrate (or ' glycan'), i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule (a glycosyl acceptor) in order to form a glycoconjugate. In biology (but not always in chemistry), glycosylation usually refers to an enzyme-catalysed reaction, whereas glycation (also 'non-enzymatic glycation' and 'non-enzymatic glycosylation') may refer to a non-enzymatic reaction (though in practice, 'glycation' often refers more specifically to Maillard-type reactions). Glycosylation is a form of co-translational and post-translational modification. Glycans serve a variety of structural and functional roles in membrane and secreted proteins. The majority of proteins synthesized in the rough endoplasmic reticulum undergo glycosylation. Glycosylation is also present in the cytoplasm and nucleus as the ''O''-GlcNAc modification. Aglycosylation is a feature of engineered antibodies to bypass glycosylation. Five clas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-linked Glycosylation
''N''-linked glycosylation, is the attachment of an oligosaccharide, a carbohydrate consisting of several sugar molecules, sometimes also referred to as glycan, to a nitrogen atom (the amide nitrogen of an asparagine (Asn) residue of a protein), in a process called ''N''-glycosylation, studied in biochemistry. This type of linkage is important for both the structure and function of many eukaryotic proteins. The ''N''-linked glycosylation process occurs in eukaryotes and widely in archaea, but very rarely in bacteria. The nature of ''N''-linked glycans attached to a glycoprotein is determined by the protein and the cell in which it is expressed. It also varies across species. Different species synthesize different types of ''N''-linked glycan. Energetics of bond formation There are two types of bonds involved in a glycoprotein: bonds between the saccharides residues in the glycan and the linkage between the glycan chain and the protein molecule. The sugar moieties are linked t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-GlcNAc
''O''-GlcNAc (short for ''O''-linked GlcNAc or ''O''-linked β-''N''-acetylglucosamine) is a reversible enzymatic post-translational modification that is found on serine and threonine residues of nucleocytoplasmic proteins. The modification is characterized by a β-glycosidic bond between the hydroxyl group of serine or threonine side chains and ''N''-acetylglucosamine (GlcNAc). ''O''-GlcNAc differs from other forms of protein glycosylation: (i) ''O''-GlcNAc is not elongated or modified to form more complex glycan structures, (ii) ''O''-GlcNAc is almost exclusively found on nuclear and cytoplasmic proteins rather than membrane proteins and secretory proteins, and (iii) ''O''-GlcNAc is a highly dynamic modification that turns over more rapidly than the proteins which it modifies. ''O''-GlcNAc is conserved across metazoans. Due to the dynamic nature of ''O''-GlcNAc and its presence on serine and threonine residues, ''O''-GlcNAcylation is similar to protein phosphorylation in some r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oligosaccharide
An oligosaccharide (/ˌɑlɪgoʊˈsækəˌɹaɪd/; from the Greek ὀλίγος ''olígos'', "a few", and σάκχαρ ''sácchar'', "sugar") is a saccharide polymer containing a small number (typically two to ten) of monosaccharides (simple sugars). Oligosaccharides can have many functions including cell recognition and cell adhesion. They are normally present as glycans: oligosaccharide chains are linked to lipids or to compatible amino acid side chains in proteins, by ''N''- or ''O''-glycosidic bonds. ''N''-Linked oligosaccharides are always pentasaccharides attached to asparagine via a beta linkage to the amine nitrogen of the side chain.. Alternately, ''O''-linked oligosaccharides are generally attached to threonine or serine on the alcohol group of the side chain. Not all natural oligosaccharides occur as components of glycoproteins or glycolipids. Some, such as the raffinose series, occur as storage or transport carbohydrates in plants. Others, such as maltodextrins or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Post-translational Modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important components in cell signaling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini. They can extend the chemical repertoire of the 20 standard amino acids by modifying an existing functional group or introducing a new one such as phosphate. Phosphorylation is a highly effective mechanism for regulating the activity of enzymes and is the most common post-translational modification. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a process called glycosyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycan
The terms glycans and polysaccharides are defined by IUPAC as synonyms meaning "compounds consisting of a large number of monosaccharides linked glycosidically". However, in practice the term glycan may also be used to refer to the carbohydrate portion of a glycoconjugate, such as a glycoprotein, glycolipid, or a proteoglycan, even if the carbohydrate is only an oligosaccharide. Glycans usually consist solely of O-glycosidic linkages of monosaccharides. For example, cellulose is a glycan (or, to be more specific, a glucan) composed of β-1,4-linked D-glucose, and chitin is a glycan composed of β-1,4-linked ''N''-acetyl-D-glucosamine. Glycans can be homo- or heteropolymers of monosaccharide residues, and can be linear or branched. Glycans and proteins Glycans can be found attached to proteins as in glycoproteins and proteoglycans. In general, they are found on the exterior surface of cells. O- and N-linked glycans are very common in eukaryotes but may also be found, although ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asparagine
Asparagine (symbol Asn or N) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain carboxamide, classifying it as a polar (at physiological pH), aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it. It is encoded by the codons AAU and AAC. History Asparagine was first isolated in 1806 in a crystalline form by French chemists Louis Nicolas Vauquelin and Pierre Jean Robiquet (then a young assistant). It was isolated from asparagus juice, in which it is abundant, hence the chosen name. It was the first amino acid to be isolated. Three years later, in 1809, Pierre Jean Robiquet identified a substance from liquorice root with properties which he qualified as very similar to those of asparagine, and which Plisson identi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology. Lipids may be broadly defined as hydrophobic or amphiphilic small molecules; the amphiphilic nature of some lipids allows them to form structures such as vesicles, multilamellar/unilamellar liposomes, or membranes in an aqueous environment. Biological lipids originate entirely or in part from two distinct types of biochemical subunits or "building-blocks": ketoacyl and isoprene groups. Using this approach, lipids may be divided into eight categories: fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids, and polyketides (derived from condensati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosyl Donor
A glycosyl donor is a carbohydrate mono- or oligosaccharide that will react with a suitable glycosyl acceptor to form a new glycosidic bond. By convention, the donor is the member of this pair that contains the resulting anomeric carbon of the new glycosidic bond.T. K. Lindhorst "Essentials of Carbohydrate Chemistry and Biochemistry" 2007 Wiley-VCH Verlag, Weinheim The resulting reaction is referred to as a glycosylation or chemical glycosylation. In a glycosyl donor, a leaving group is required at the anomeric position. The simplest leaving group is the OH group that is naturally present in monosaccharides, but it requires activation by acid catalysis in order to function as leaving group (in the Fischer glycosylation). More effective leaving groups are in general used in the glycosyl donors employed in chemical synthesis of glycosides. Typical leaving groups are halides, thioalkyl groups, or imidates, but acetate, phosphate, and O-pentenyl groups are also employed. Natural glycosyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glypiation
Glypiation is the addition by covalent bonding of a glycosylphosphatidylinositol (GPI) anchor and is a common post-translational modification that localizes proteins to cell membranes. This special kind of glycosylation is widely detected on surface glycoproteins in eukaryotes and some Archaea. GPI anchors consist of a phosphoethanolamine linker that binds to the C-terminus of target proteins. Glycan's core structure has a phospholipid tail that anchors the structure to the membrane. Both the lipid moiety of the tail and the sugar residues in the glycan core has considerable variation, demonstrating vast functional diversity that includes signal transduction, cell adhesion and immune recognition. GPI anchors can also be cleaved by enzymes such as phospholipase C to regulate the localization of proteins that are anchored at the plasma membrane. Mechanism Similar to the precursor glycan used for N-glycosylation, GPI anchor biosynthesis begins on the cytoplasmic leaflet of the ER and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosyl Acceptor
A glycosyl acceptor is any suitable nucleophile-containing molecule that will react with a glycosyl donor to form a new glycosidic bond. By convention, the acceptor is the member of this pair which did not contain the resulting anomeric carbon of the new glycosidic bond. Since the nucleophilic atom of the acceptor is typically an oxygen atom, this can be remembered using the mnemonic of ''the acceptor is the alcohol.'' A glycosyl acceptor can be a mono- or oligosaccharide that contains an available nucleophile, such as an unprotected hydroxyl. Background Examples glucose to haemoglobin See also * Chemical glycosylation * Glycosyl halide * Armed and disarmed saccharides * Carbohydrate chemistry Carbohydrate chemistry is a subdiscipline of chemistry primarily concerned with the detection, synthesis, structure, and function of carbohydrates. Due to the general structure of carbohydrates, their synthesis is often preoccupied with the select ... References *{{cite journal , las ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycation
Glycation (sometimes called non-enzymatic glycosylation) is the covalent attachment of a sugar to a protein or lipid. Typical sugars that participate in glycation are glucose, fructose, and their derivatives. Glycation is the non-enzymatic process responsible for many (e.g. micro and macrovascular) complications in diabetes mellitus and is implicated in some diseases and in aging. Glycation end products are believed to play a causative role in the vascular complications of diabetes mellitus. In contrast with glycation, glycosylation is the enzyme-mediated ATP-dependent attachment of sugars to protein or lipid. Glycosylation occurs at defined sites on the target molecule. It is a common form of post-translational modification of proteins and is required for the functioning of the mature protein. Biochemistry Glycations occur mainly in the bloodstream to a small proportion of the absorbed simple sugars: glucose, fructose, and galactose. It appears that fructose has approximately ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
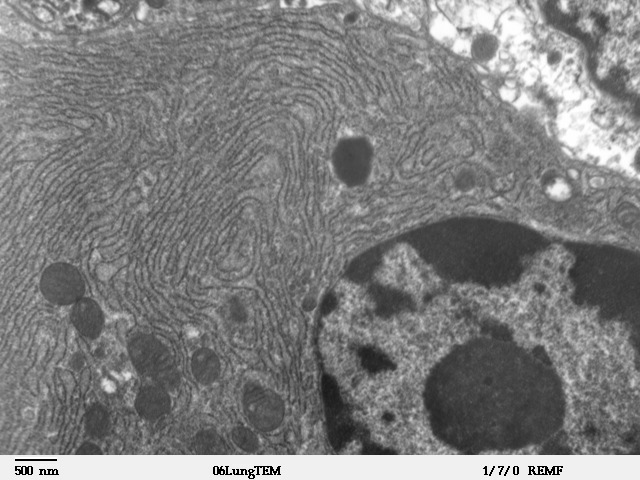
Rough Endoplasmic Reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa. The two types of ER share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of cells contain different ratios of the two types of ER depending on the activities of the cell. RER is found mainly toward the nucleus of cell and SER towards the cell membrane or plasma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |