|
Glycosyl Acceptor
A glycosyl acceptor is any suitable nucleophile-containing molecule that will react with a glycosyl donor to form a new glycosidic bond. By convention, the acceptor is the member of this pair which did not contain the resulting anomeric carbon of the new glycosidic bond. Since the nucleophilic atom of the acceptor is typically an oxygen atom, this can be remembered using the mnemonic of ''the acceptor is the alcohol.'' A glycosyl acceptor can be a mono- or oligosaccharide that contains an available nucleophile, such as an unprotected hydroxyl. Background Examples glucose to haemoglobin See also * Chemical glycosylation * Glycosyl halide * Armed and disarmed saccharides * Carbohydrate chemistry Carbohydrate chemistry is a subdiscipline of chemistry primarily concerned with the detection, synthesis, structure, and function of carbohydrates. Due to the general structure of carbohydrates, their synthesis is often preoccupied with the select ... References *{{cite journal , las ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. History The terms ''nucleophile'' and ''electrophile'' were introduced by Christopher Kelk Ingold in 1933, replacing the terms ''anionoid'' and ''cationoid' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosyl Donor
A glycosyl donor is a carbohydrate mono- or oligosaccharide that will react with a suitable glycosyl acceptor to form a new glycosidic bond. By convention, the donor is the member of this pair that contains the resulting anomeric carbon of the new glycosidic bond.T. K. Lindhorst "Essentials of Carbohydrate Chemistry and Biochemistry" 2007 Wiley-VCH Verlag, Weinheim The resulting reaction is referred to as a glycosylation or chemical glycosylation. In a glycosyl donor, a leaving group is required at the anomeric position. The simplest leaving group is the OH group that is naturally present in monosaccharides, but it requires activation by acid catalysis in order to function as leaving group (in the Fischer glycosylation). More effective leaving groups are in general used in the glycosyl donors employed in chemical synthesis of glycosides. Typical leaving groups are halides, thioalkyl groups, or imidates, but acetate, phosphate, and O-pentenyl groups are also employed. Natural glycosyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosidic Bond
A glycosidic bond or glycosidic linkage is a type of covalent bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate. A glycosidic bond is formed between the hemiacetal or hemiketal group of a saccharide (or a molecule derived from a saccharide) and the hydroxyl group of some compound such as an alcohol. A substance containing a glycosidic bond is a glycoside. The term 'glycoside' is now extended to also cover compounds with bonds formed between hemiacetal (or hemiketal) groups of sugars and several chemical groups other than hydroxyls, such as -SR (thioglycosides), -SeR (selenoglycosides), -NR1R2 (N-glycosides), or even -CR1R2R3 (C-glycosides). Particularly in naturally occurring glycosides, the compound ROH from which the carbohydrate residue has been removed is often termed the aglycone, and the carbohydrate residue itself is sometimes referred to as the 'glycone'. S-, N-, C-, and O-glycosidic bonds Glycosidi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anomer
In carbohydrate chemistry, a pair of anomers () is a pair of near-identical stereoisomers that differ at only the anomeric carbon, the carbon that bears the aldehyde or ketone functional group in the sugar's open-chain form. However, in order for anomers to exist, the sugar must be in its cyclic form, since in open-chain form, the anomeric carbon is planar and thus achiral. More formally stated, then, an anomer is an epimer at the hemiacetal/hemiketal carbon in a cyclic saccharide. Anomerization is the process of conversion of one anomer to the other. As is typical for stereoisomeric compounds, different anomers have different physical properties, melting points and specific rotations. Nomenclature Two anomers are designated alpha (α) or beta (β), according to the configurational relationship between the ''anomeric centre'' and the ''anomeric reference atom'', hence they are relative stereodescriptors. The anomeric centre in hemiacetals is the anomeric carbon C-1; in hemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Glycosylation
A chemical glycosylation reaction involves the coupling of a glycosyl donor, to a glycosyl acceptor forming a glycoside. If both the donor and acceptor are sugars, then the product is an oligosaccharide. The reaction requires activation with a suitable activating reagent. The reactions often result in a mixture of products due to the creation of a new stereogenic centre at the anomeric position of the glycosyl donor. The formation of a glycosidic linkage allows for the synthesis of complex polysaccharides which may play important roles in biological processes and pathogenesis and therefore having synthetic analogs of these molecules allows for further studies with respect to their biological importance. Terminology The glycosylation reaction involves the coupling of a glycosyl donor and a glycosyl acceptor via initiation using an activator under suitable reaction conditions. * A glycosyl donor is a sugar with a suitable leaving group at the anomeric position. This group, u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosyl Halide
A glycosyl group is a univalent free radical or substituent structure obtained by removing the hemiacetal hydroxyl group from the cyclic form of a monosaccharide and, by extension, of a lower oligosaccharide. Glycosyl also reacts with inorganic acids, such as phosphoric acid, forming an ester such as glucose 1-phosphate. Examples In cellulose, glycosyl groups link together 1,4-β-D-glucosyl units to form chains of (1,4-β-D-glucosyl)n. Other examples include ribityl in 6,7-Dimethyl-8-ribityllumazine, and glycosylamines. Alternative substituent groups Instead of the hemiacetal hydroxyl group, a ''hydrogen'' atom can be removed to form a substituent, for example the hydrogen from the C3 hydroxyl of a glucose molecule. Then the substituent is called D-glucopyranos-3-''O''-yl as it appears in the name of the drug Mifamurtide. Recent detection of the Au3+ in living organism was possible through the use of C-glycosyl pyrene, where it's permeability through cell membrane and fluor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
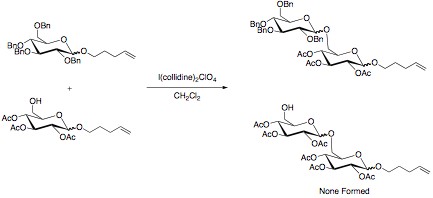
Armed And Disarmed Saccharides
The armed/disarmed approach to glycosylation is an effective way to prevent sugar molecules from self-glycosylation when synthesizing disaccharides. This approach was first recognized when acetylated sugars only acted as glycosyl acceptors when reacted with benzylated sugars. The acetylated sugars were termed “disarmed” while the benzylated sugars were termed “armed”. Electronic Effect The selectivity in the reaction is due to the stronger electron withdrawing power of the esters compared to the ethers. A stronger electron withdrawing substituent leads to a greater destabilization of the oxocarbenium ion. This slows this reaction pathway, and allows for disaccharide formation to occur with the benzylated sugar. Other effective electron withdrawing groups that have shown selectivity are halogens and azido groups, while deoxygenation has been proven an effective tool in “arming” sugars. Torsional Effect Disarming sugars can also be accomplished by adding 1,3-dioxane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbohydrate Chemistry
Carbohydrate chemistry is a subdiscipline of chemistry primarily concerned with the detection, synthesis, structure, and function of carbohydrates. Due to the general structure of carbohydrates, their synthesis is often preoccupied with the selective formation of glycosidic linkages and the selective reaction of hydroxyl groups; as a result, it relies heavily on the use of protecting groups. Monosaccharides Individual saccharide residues are termed monosaccharides. Carbohydrate synthesis Carbohydrate synthesis is a sub-field of organic chemistry concerned specifically with the generation of natural and unnatural carbohydrate structures. This can include the synthesis of monosaccharide residues or structures containing more than one monosaccharide, known as oligosaccharides. Glycosidic bond formation * Chemical glycosylation * Fischer glycosidation * Glycosyl halide * Koenigs-Knorr reaction Protecting groups * Carbohydrate acetalisation * Trimethylsilyl * Benzyl Ether * para ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |