glycosyl donor A glycosyl donor is a carbohydrate mono- or oligosaccharide that will react with a suitable glycosyl acceptor to form a new glycosidic bond. By convention, the donor is the member of this pair that contains the resulting anomeric carbon of the new ...
, to a glycosyl acceptor A glycosyl acceptor is any suitable nucleophile-containing molecule that will react with a glycosyl donor to form a new glycosidic bond. By convention, the acceptor is the member of this pair which did not contain the resulting anomeric carbon of th ...
forming a glycoside
In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycoside ...
. If both the donor and acceptor are sugars, then the product is an oligosaccharide
An oligosaccharide (/ˌɑlɪgoʊˈsækəˌɹaɪd/; from the Greek ὀλίγος ''olígos'', "a few", and σάκχαρ ''sácchar'', "sugar") is a saccharide polymer containing a small number (typically two to ten) of monosaccharides (simple sug ...
. The reaction requires activation with a suitable activating reagent. The reactions often result in a mixture of products due to the creation of a new stereogenic centre at the anomeric position of the glycosyl donor. The formation of a glycosidic linkage
A glycosidic bond or glycosidic linkage is a type of covalent bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.
A glycosidic bond is formed between the hemiacetal or hemiketal grou ...
allows for the synthesis of complex polysaccharides
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with w ...
which may play important roles in biological processes and pathogenesis
Pathogenesis is the process by which a disease or disorder develops. It can include factors which contribute not only to the onset of the disease or disorder, but also to its progression and maintenance. The word comes from Greek πάθος ''pat ...
and therefore having synthetic analogs of these molecules allows for further studies with respect to their biological importance.
Terminology
The glycosylation reaction involves the coupling of a glycosyl donor and a glycosyl acceptor via initiation using an activator under suitable reaction conditions. * Aglycosyl donor A glycosyl donor is a carbohydrate mono- or oligosaccharide that will react with a suitable glycosyl acceptor to form a new glycosidic bond. By convention, the donor is the member of this pair that contains the resulting anomeric carbon of the new ...
is a sugar with a suitable leaving group at the anomeric position. This group, under the reaction conditions, is activated and via the formation of an oxocarbenium
An oxocarbenium ion (or oxacarbenium ion) is a chemical species characterized by a central sp2-hybridized carbon, an oxygen substituent, and an overall positive charge that is delocalized between the central carbon and oxygen atoms. An oxocarbenium ...
is eliminated leaving an electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that car ...
anomeric carbon.
* A glycosyl acceptor A glycosyl acceptor is any suitable nucleophile-containing molecule that will react with a glycosyl donor to form a new glycosidic bond. By convention, the acceptor is the member of this pair which did not contain the resulting anomeric carbon of th ...
is a sugar with an unprotected nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
hydroxyl group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
which may attack the carbon of the oxocarbenium ion formed during the reaction and allow for the formation of the glycosidic bond.
An activator is commonly a Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
which enables the leaving group at the anomeric position to leave and results in the formation of the oxocarbenium ion.
Stereochemistry
The formation of a glycosidic linkage results in the formation of a new stereogenic centre and therefore a mixture of products may be expected to result. The linkage formed may either be axial or equatorial (α or β with respect to glucose). To better understand this, the mechanism of a glycosylation reaction must be considered.
Neighbouring group participation
The stereochemical outcome of a glycosylation reaction may in certain cases be affected by the type of protecting group employed at position 2 of the glycosyl donor. A participating group, typically one with a carboxyl group present, will predominantly result in the formation of a β-glycoside. Whereas a non-participating group, a group usually without a carboxyl group, will often result in an α-glycoside. Below it can be seen that having an acetyl protecting group at position 2 allows for the formation for an acetoxonium ion intermediate that blocks attack to the bottom face of the ring therefore allowing for the formation of the β-glycoside predominantly. Alternatively, the absence of a participating group at position 2 allows for either attack from the bottom or top face. Since the α-glycoside product will be favoured by the
Alternatively, the absence of a participating group at position 2 allows for either attack from the bottom or top face. Since the α-glycoside product will be favoured by the anomeric effect
In organic chemistry, the anomeric effect or Edward-Lemieux effect is a stereoelectronic effect that describes the tendency of heteroatomic substituents adjacent to a heteroatom within a cyclohexane ring to prefer the ''axial'' orientation instea ...
, the α-glycoside usually predominates.

Protecting groups
Differentprotecting groups
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In man ...
on either the glycosyl donor or the glycosyl acceptor may affect the reactivity and yield of the glycosylation reaction. Typically, electron-withdrawing group
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of th ...
s such as acetyl or benzoyl groups are found to decrease the reactivity of the donor/acceptor and are therefore termed "disarming" groups. Electron-donating groups such as the benzyl group, are found to increase the reactivity of the donor/acceptor and are therefore called "arming" groups.
Current methods in glycoside synthesis
Glycosyl iodides
Glycosyl iodides were first introduced for use in glycosylation reactions in 1901 by Koenigs and Knorr although were often considered too reactive for synthetic use. Recently several research groups have shown these donors to have unique reactive properties and can differ from other glycosyl chlorides or bromides with respect to reaction time, efficiency, andstereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereo ...
. Glycosyl iodides may be made under a variety of conditions, one method of note is the reaction of a 1-''O''-acetylpyranoside with TMSI.
 Iodide donors may typically be activated under basic conditions to give β-glycosides with good selectivity. The use of tetraalkylammonium iodide salts such as tetrabutylammonium iodide ( TBAI) allows for in-situ
Iodide donors may typically be activated under basic conditions to give β-glycosides with good selectivity. The use of tetraalkylammonium iodide salts such as tetrabutylammonium iodide ( TBAI) allows for in-situ anomerization
In carbohydrate chemistry, a pair of anomers () is a pair of near-identical stereoisomers that differ at only the anomeric carbon, the carbon that bears the aldehyde or ketone functional group in the sugar's open-chain form. However, in order fo ...
of the α-glycosyl halide to the β-glycosyl halide and provides the α-glycoside in good selectivity.

Thioglycosides
Thioglycosides were first reported in 1909 byFischer
Fischer is a German occupational surname, meaning fisherman. The name Fischer is the fourth most common German surname. The English version is Fisher.
People with the surname A
* Abraham Fischer (1850–1913) South African public official
* ...
and since then have been explored constantly allowing for the development of numerous protocols for their preparation.
The advantage of using thioglycosides is their stability under a wide range of reaction conditions allowing for protecting group manipulations. Additionally thioglycosides act as temporary protecting groups at the anomeric position allowing for thioglycosides to be useful as both glycosyl donors as well as glycosyl acceptors.
Thioglycosides are usually prepared by reacting per-acetylated sugars with and the appropriate thiol.
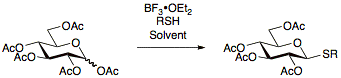 Thioglycosides used in glycosylation reactions as donors can be activated under a wide range of conditions, most notably using NIS/AgOTf.
Thioglycosides used in glycosylation reactions as donors can be activated under a wide range of conditions, most notably using NIS/AgOTf.

Trichloroacetimidates
Trichloroacetimidates were first introduced and explored by Schmidt in 1980 and since then have become very popular for glycoside synthesis. The use of trichloroacetimidates provides many advantages including ease of formation, reactivity and stereochemical outcome. ''O''-Glycosyl trichloroacetimidates are prepared via the addition of trichloroacetonitrile () under basic conditions to a free anomeric hydroxyl group. Typical activating groups for glycosylation reactions using trichloroacetimidates are or TMSOTf.
Typical activating groups for glycosylation reactions using trichloroacetimidates are or TMSOTf.
 Column chromatographic purification of the reaction mixture can sometimes be challenging due to the trichloroacetamide by-product. This can, however, be overcome by washing the organic layer with 1 M NaOH solution in a separatory funnel prior to chromatography. Acetyl protecting groups were found to be stable during this procedure.
Column chromatographic purification of the reaction mixture can sometimes be challenging due to the trichloroacetamide by-product. This can, however, be overcome by washing the organic layer with 1 M NaOH solution in a separatory funnel prior to chromatography. Acetyl protecting groups were found to be stable during this procedure.
Notable synthetic products
Below are a few examples of some notable targets obtained via a series of glycosylation reactions.

See also
*Glycosylation
Glycosylation is the reaction in which a carbohydrate (or 'glycan'), i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule (a glycosyl acceptor) in order to form a glycoconjugate. In biology (but not ...
* Glycosyl transferase
Glycosyltransferases (GTFs, Gtfs) are enzymes (EC 2.4) that establish natural glycosidic linkages. They catalyze the transfer of saccharide moieties from an activated nucleotide sugar (also known as the " glycosyl donor") to a nucleophilic ...
* Glycorandomization
* Glycation
Glycation (sometimes called non-enzymatic glycosylation) is the covalent attachment of a sugar to a protein or lipid. Typical sugars that participate in glycation are glucose, fructose, and their derivatives. Glycation is the non-enzymatic proces ...
* Carbohydrate chemistry
Carbohydrate chemistry is a subdiscipline of chemistry primarily concerned with the detection, synthesis, structure, and function of carbohydrates. Due to the general structure of carbohydrates, their synthesis is often preoccupied with the selec ...
* Oligosaccharide
An oligosaccharide (/ˌɑlɪgoʊˈsækəˌɹaɪd/; from the Greek ὀλίγος ''olígos'', "a few", and σάκχαρ ''sácchar'', "sugar") is a saccharide polymer containing a small number (typically two to ten) of monosaccharides (simple sug ...
* Carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or ...
References
Books
*{{cite book , first=Marco , last=Brito-Arias , title=Synthesis and Characterization of Glycosides , publisher=Springer , edition=3rd , date=2022 , isbn=978-3-030-97854-9 , doi=10.1007/978-3-030-97854-9, s2cid=248302952 Carbohydrates Carbohydrate chemistry