|
Polysaccharides
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long-chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with water (hydrolysis) using amylase enzymes as catalyst, which produces constituent sugars (monosaccharides or oligosaccharides). They range in structure from linear to highly branched. Examples include storage polysaccharides such as starch, glycogen and galactogen and structural polysaccharides such as hemicellulose and chitin. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure, these macromolecules can have distinct properties from their monosaccharide building blocks. They may be amorphous or even insoluble in water. When all the monosaccharides in a polysaccharide are the same type, the polysaccharide is called a homopolysaccharide or homoglycan, but when more t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemicellulose
A hemicellulose (also known as polyose) is one of a number of heteropolymers (matrix polysaccharides), such as arabinoxylans, present along with cellulose in almost all embryophyte, terrestrial plant cell walls. Cellulose is crystalline, strong, and resistant to hydrolysis. Hemicelluloses are branched, shorter in length than cellulose, and also show a propensity to crystallize. They can be hydrolyzed by dilute acid or Base (chemistry), base as well as a myriad of Cellulase, hemicellulase enzymes. Composition Diverse kinds of hemicelluloses are known. Important examples include xylan, glucuronoxylan, arabinoxylan, glucomannan, and xyloglucan. Hemicelluloses are polysaccharides often associated with cellulose, but with distinct compositions and structures. Whereas cellulose is derived exclusively from glucose, hemicelluloses are composed of diverse sugars, and can include the five-carbon sugars xylose and arabinose, the six-carbon sugars glucose, mannose and galactose, and the si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
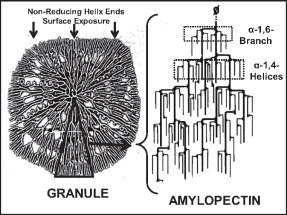
Amylopectin
Amylopectin is a water-insoluble polysaccharide and highly branched polymer of α-glucose units found in plants. It is one of the two components of starch, the other being amylose. Plants store starch within specialized organelles called amyloplasts. To generate energy, the plant hydrolyzes the starch, releasing the glucose subunits. Humans and other animals that eat plant foods also use amylase, an enzyme that assists in breaking down amylopectin, to initiate the hydrolysis of starch. Starch is made of about 70–80% amylopectin by weight, though it varies depending on the source. For example, it ranges from lower percent content in long-grain rice, amylomaize, and Russet Burbank potato, russet potatoes to 100% in glutinous rice, waxy potato starch, and waxy corn. Amylopectin is highly branched, being formed of 2,000 to 200,000 glucose units. Its inner chains are formed of 20–24 glucose subunits. Starch gelatinization, Dissolved amylopectin starch has a lower tendency of retrog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbohydrate
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' may differ). This formula does not imply direct covalent bonding between hydrogen and oxygen atoms; for example, in , hydrogen is covalently bonded to carbon, not oxygen. While the 2:1 hydrogen-to-oxygen ratio is characteristic of many carbohydrates, exceptions exist. For instance, uronic acids and deoxy-sugars like fucose deviate from this precise stoichiometric definition. Conversely, some compounds conforming to this definition, such as formaldehyde and acetic acid, are not classified as carbohydrates. The term is predominantly used in biochemistry, functioning as a synonym for saccharide (), a group that includes sugars, starch, and cellulose. The saccharides are divided into four chemical groups: monosaccharides, disaccharides, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biopolymer
Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, classified according to the monomers used and the structure of the biopolymer formed: polynucleotides, polypeptides, and polysaccharides. The Polynucleotides, RNA and DNA, are long polymers of nucleotides. Polypeptides include proteins and shorter polymers of amino acids; some major examples include collagen, actin, and fibrin. Polysaccharides are linear or branched chains of sugar carbohydrates; examples include starch, cellulose, and alginate. Other examples of biopolymers include natural rubbers (polymers of isoprene), suberin and lignin (complex polyphenolic polymers), cutin and cutan (complex polymers of long-chain fatty acids), melanin, and polyhydroxyalkanoates (PHAs). In addition to their many essential roles in living ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight. It is used by plants to make cellulose, the most abundant carbohydrate in the world, for use in cell walls, and by all living Organism, organisms to make adenosine triphosphate (ATP), which is used by the cell as energy. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as amylose and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form is -glucose, while its Stereoisomerism, stereoisomer L-glucose, -glucose is produced synthetically in comparatively small amounts and is less biologicall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chitin
Chitin (carbon, C8hydrogen, H13oxygen, O5nitrogen, N)n ( ) is a long-chain polymer of N-Acetylglucosamine, ''N''-acetylglucosamine, an amide derivative of glucose. Chitin is the second most abundant polysaccharide in nature (behind only cellulose); an estimated 1 billion tons of chitin are produced each year in the biosphere. It is a primary component of cell walls in fungi (especially filamentous and mushroom-forming fungi), the exoskeletons of arthropods such as crustaceans and insects, the radulae, cephalopod beaks and Gladius (cephalopod), gladii of molluscs and in some nematodes and diatoms. It is also synthesised by at least some fish and lissamphibians. Commercially, chitin is extracted from the shells of crabs, shrimps, shellfish and lobsters, which are major by-products of the seafood industry. The structure of chitin is comparable to cellulose, forming crystalline nanofibrils or whiskers. It is functionally comparable to the protein keratin. Chitin has proved useful ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homopolysaccharide
Homopolysaccharides are polysaccharides composed of a single type of sugar monomer. For example, cellulose is an unbranched homopolysaccharide made up of glucose monomers connected via beta-glycosidic linkages; glycogen is a branched form, where the glucose monomers are joined by alpha-glycosidic linkages. Depending upon the molecules attached that are of the following types: # Glucan - A polysaccharide of glucose # Fructan - A polysaccharide of fructose # Galactan - A polysaccharide of galactose # Arabinan - A polysaccharide of arabinose # Xylan - A polysaccharide of xylose Xylose ( , , "wood") is a sugar first isolated from wood, and named for it. Xylose is classified as a monosaccharide of the aldopentose type, which means that it contains five carbon atoms and includes an aldehyde functional group. It is deriv ...Champe, Harvey, Ferrier. Biochemistry 4th Edition. 2008. 90. References Polysaccharides {{polymer-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cellulose
Cellulose is an organic compound with the chemical formula, formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of glycosidic bond, β(1→4) linked glucose, D-glucose units. Cellulose is an important structural component of the primary cell wall of green plants, many forms of algae and the oomycetes. Some species of bacteria secrete it to form biofilms. Cellulose is the most abundant biopolymer, organic polymer on Earth. The cellulose content of cotton fibre is 90%, that of wood is 40–50%, and that of dried hemp is approximately 57%. Cellulose is mainly used to produce paperboard and paper. Smaller quantities are converted into a wide variety of derivative products such as cellophane and rayon. Conversion of cellulose from energy crops into biofuels such as cellulosic ethanol is under development as a renewable fuel source. Cellulose for industrial use is mainly obtained from wood pulp and cotton. Cellulose is also greatly affected by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of Biomolecule, biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
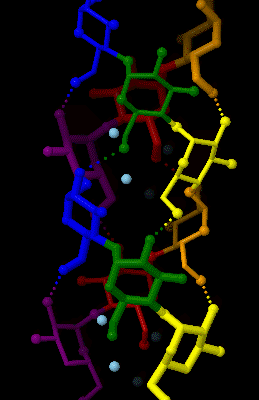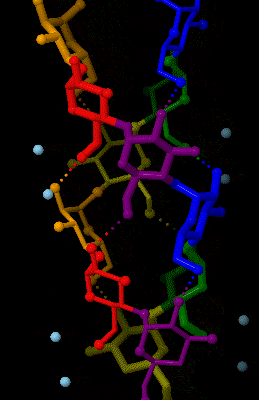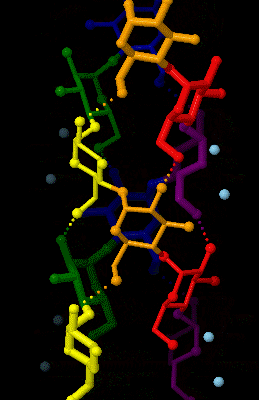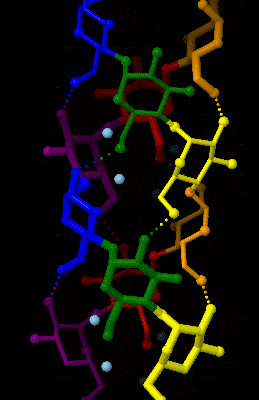
Amylose
Amylose is a polysaccharide made of α-D-glucose units, bonded to each other through α(1→4) glycosidic bonds. It is one of the two components of starch, making up approximately 20–25% of it. Because of its tightly packed Helix, helical structure, amylose is more resistant to digestion than other starch molecules and is therefore an important form of resistant starch. Structure Amylose is made up of α(1→4) bound glucose molecules. The carbon atoms on glucose are numbered, starting at the aldehyde (C=O) carbon, so, in amylose, the 1-carbon on one glucose molecule is linked to the 4-carbon on the next glucose molecule (α(1→4) bonds). The structural formula of amylose is pictured at right. The number of repeated glucose subunits (n) is usually in the range of 300 to 3000, but can be many thousands. There are three main forms of amylose chains can take. It can exist in a disordered amorphous conformation, found both in starch granules and in hydrated amylose (when starch i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amylose 3Dprojection
Amylose is a polysaccharide made of α-D-glucose units, bonded to each other through α(1→4) glycosidic bonds. It is one of the two components of starch, making up approximately 20–25% of it. Because of its tightly packed helical structure, amylose is more resistant to digestion than other starch molecules and is therefore an important form of resistant starch. Structure Amylose is made up of α(1→4) bound glucose molecules. The carbon atoms on glucose are numbered, starting at the aldehyde (C=O) carbon, so, in amylose, the 1-carbon on one glucose molecule is linked to the 4-carbon on the next glucose molecule (α(1→4) bonds). The structural formula of amylose is pictured at right. The number of repeated glucose subunits (n) is usually in the range of 300 to 3000, but can be many thousands. There are three main forms of amylose chains can take. It can exist in a disordered amorphous conformation, found both in starch granules and in hydrated amylose (when starch is coo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macromolecule
A macromolecule is a "molecule of high relative molecular mass, the structure of which essentially comprises the multiple repetition of units derived, actually or conceptually, from molecules of low relative molecular mass." Polymers are physical examples of macromolecules. Common macromolecules are biopolymers (nucleic acids, proteins, and carbohydrates). and polyolefins (polyethylene) and polyamides (nylon). Synthetic macromolecules Many macromolecules are synthetic polymers (plastics, synthetic fibers, and synthetic rubber. Polyethylene is produced on a particularly large scale such that ethylene is the primary product in the chemical industry. Macromolecules in nature * Proteins are polymers of amino acids joined by peptide bonds. * DNA and RNA are polymers of nucleotides joined by phosphodiester bonds. These nucleotides consist of a phosphate group, a sugar ( ribose in the case of RNA, deoxyribose in the case of DNA), and a nucleotide base (either adenine, guan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |