|
Flow Batteries
A flow battery, or redox flow battery (after reduction–oxidation), is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids that are pumped through the system on separate sides of a membrane. Ion transfer inside the cell (accompanied by flow of electric current through an external circuit) occurs through the membrane while both liquids circulate in their own respective space. Cell voltage is chemically determined by the Nernst equation and ranges, in practical applications, from 1.0 to 2.43 volts. The energy capacity is a function of the electrolyte volume and the power is a function of the surface area of the electrodes. A flow battery may be used like a fuel cell (where the spent fuel is extracted and new fuel is added to the system) or like a rechargeable battery (where an electric power source drives regeneration of the fuel). While flow batteries have certain technical advantages over conventional rechargeabl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox Flow Battery
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogenation, C=C (and other) bonds ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dalian
Dalian () is a major sub-provincial port city in Liaoning province, People's Republic of China, and is Liaoning's second largest city (after the provincial capital Shenyang) and the third-most populous city of Northeast China. Located on the southern tip of Liaodong peninsula, it is the southernmost city in both Liaoning and the entire Northeast. Dalian borders the prefectural cities of Yingkou and Anshan to the north and Dandong to the northeast, and also shares maritime boundaries with Qinhuangdao and Huludao across the Liaodong Bay to west and northwest, Yantai and Weihai on the Shandong peninsula across the Bohai Strait to the south, and North Korea across the Korea Bay to the east. As of the 2020 census, its total population was 7,450,785 inhabitants whom 5,106,719 lived in the built-up (or metro) area made of 6 out of 7 urban districts, Pulandian District not being conurbated yet. Today a financial, shipping, and logistics center for East Asia, Dalian has a signific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secondary Battery
A rechargeable battery, storage battery, or secondary cell (formally a type of energy accumulator), is a type of electrical battery which can be charged, discharged into a load, and recharged many times, as opposed to a disposable or primary battery, which is supplied fully charged and discarded after use. It is composed of one or more electrochemical cells. The term "accumulator" is used as it accumulates and stores energy through a reversible electrochemical reaction. Rechargeable batteries are produced in many different shapes and sizes, ranging from button cells to megawatt systems connected to stabilize an electrical distribution network. Several different combinations of electrode materials and electrolytes are used, including lead–acid, zinc–air, nickel–cadmium (NiCd), nickel–metal hydride (NiMH), lithium-ion (Li-ion), lithium iron phosphate (LiFePO4), and lithium-ion polymer (Li-ion polymer). Rechargeable batteries typically initially cost more than ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Regenerative Fuel Cell
A regenerative fuel cell or reverse fuel cell (RFC) is a fuel cell run in reverse mode, which consumes electricity and chemical B to produce chemical A. By definition, the process of any fuel cell could be reversed. However, a given device is usually optimized for operating in one mode and may not be built in such a way that it can be operated backwards. Standard fuel cells operated backwards generally do not make very efficient systems unless they are purpose-built to do so as with high-pressure electrolysers, regenerative fuel cells, solid-oxide electrolyser cells and unitized regenerative fuel cells. Process description A hydrogen fueled proton exchange membrane fuel cell, for example, uses hydrogen gas (H2) and oxygen (O2) to produce electricity and water (H2O); a regenerative hydrogen fuel cell uses electricity and water to produce hydrogen and oxygen. When the fuel cell is operated in regenerative mode, the anode for the electricity production mode (fuel cell mode) becomes th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cooperative Patent Classification
The Cooperative Patent Classification (CPC) is a patent classification system, which has been jointly developed by the European Patent Office (EPO) and the United States Patent and Trademark Office (USPTO).CPC replaces ECLA on Espacenet , issue 4/2012, December 2012, p. 4. The CPC is substantially based on the previous European classification system (ECLA), which itself was a more specific and detailed version of the |
Electrochemical Engineering
Electrochemical engineering is the branch of chemical engineering dealing with the technological applications of electrochemical phenomena, such as electrosynthesis of chemicals, electrowinning and refining of metals, flow batteries and fuel cells, surface modification by electrodeposition, electrochemical separations and corrosion. This discipline is an overlap between electrochemistry and chemical engineering. According with the IUPAC, the term ''electrochemical engineering'' is reserved for electricity intensive processes for industrial or energy storage applications, and should not be confused with ''applied electrochemistry'', which comprises small batteries, amperometric sensors, microfluidic devices, microelectrodes, solid-state devices, voltammetry at disc electrodes, etc. More than 6% of the electricity is consumed by large-scale electrochemical operations in the US. Scope Electrochemical engineering combines the study of heterogeneous charge transfer at electrode/ele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Power Density
Power density is the amount of power (time rate of energy transfer) per unit volume. In energy transformers including batteries, fuel cells, motors, power supply units etc., power density refers to a volume, where it is often called volume power density, expressed as W/m3. In reciprocating internal combustion engines, power density (power per swept volume or brake horsepower per cubic centimeter) is an important metric, based on the ''internal'' capacity of the engine, not its external size. Examples See also *Surface power density, energy per unit of area *Energy density, energy per unit volume * Specific energy, energy per unit mass * Power-to-weight ratio/specific power, power per unit mass **Specific absorption rate Specific absorption rate (SAR) is a measure of the rate at which energy is absorbed per unit mass by a human body when exposed to a radio frequency (RF) electromagnetic field. It can also refer to absorption of other forms of energy by tissue, inc ... ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
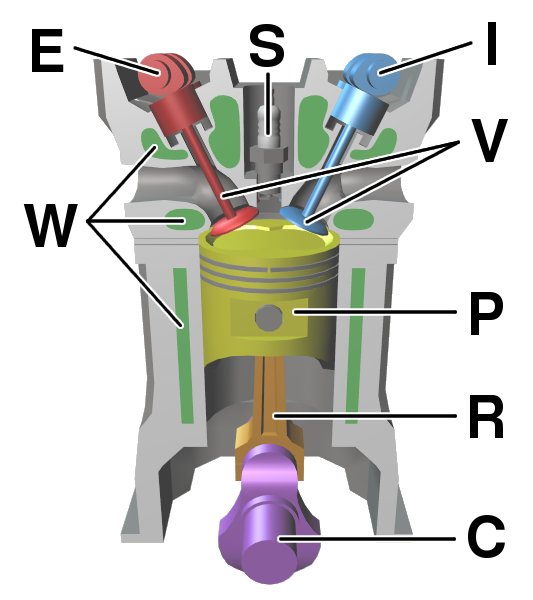
Internal Combustion Engine
An internal combustion engine (ICE or IC engine) is a heat engine in which the combustion of a fuel occurs with an oxidizer (usually air) in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal combustion engine, the expansion of the high-temperature and high-pressure gases produced by combustion applies direct force to some component of the engine. The force is typically applied to pistons ( piston engine), turbine blades (gas turbine), a rotor (Wankel engine), or a nozzle ( jet engine). This force moves the component over a distance, transforming chemical energy into kinetic energy which is used to propel, move or power whatever the engine is attached to. This replaced the external combustion engine for applications where the weight or size of an engine was more important. The first commercially successful internal combustion engine was created by Étienne Lenoir around 1860, and the first modern internal combustion engine, known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a fluid (the ''absorbate'') is dissolved by or permeates a liquid or solid (the ''absorbent''). Adsorption is a '' surface phenomenon'', while absorption involves the whole volume of the material, although adsorption does often precede absorption. The term ''sorption'' encompasses both processes, while ''desorption'' is the reverse of it. Like surface tension, adsorption is a consequence of surface energy. In a bulk material, all the bonding requirements (be they ionic, covalent or metallic) of the constituent atoms of the material are fulfilled by other atoms in the material. However, atoms on the surface of the adsorbent are not wholly surrounded by other adsorbent atoms and therefore can attract adsorbates. The exact nature of the bon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electricity
Electricity is the set of physical phenomena associated with the presence and motion of matter that has a property of electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by Maxwell's equations. Various common phenomena are related to electricity, including lightning, static electricity, electric heating, electric discharges and many others. The presence of an electric charge, which can be either positive or negative, produces an electric field. The movement of electric charges is an electric current and produces a magnetic field. When a charge is placed in a location with a non-zero electric field, a force will act on it. The magnitude of this force is given by Coulomb's law. If the charge moves, the electric field would be doing work on the electric charge. Thus we can speak of electric potential at a certain point in space, which is equal to the work done by an external agent in carrying a unit of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also function as electrolytes. El ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |





.jpg)