|
Flavagline
Flavaglines are a family of natural products that are found in plants of the genus ''Aglaia'' (Meliaceae). These compounds are characterized by a cyclopenta[''b'']benzofuran skeleton. In 1982 King and colleagues discovered the first member of this family, rocaglamide, based on its antileukemic activity. Since then, about 50 other flavaglines have been characterized. These molecules display strong insecticidal, antifungal, anti-inflammatory, neuroprotective, cardioprotective and anticarcinogen, anticancer activities. In mouse models of cancer, flavaglines enhance the efficacy of chemotherapies and also alleviate the cardiac adverse effect of these chemotherapies. The challenge raised by their structural complexity has attracted the attention of some organic chemists. In 1990, Barry Trost presented an Enantiomer, enantioselective synthesis of rocaglamide in 18 steps and confirmed its absolute configuration. See also * FL3 (flavagline) *Rocaglamide *Silvestrol *Aglafoline Referenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavagline FL3
Flavaglines are a family of natural products that are found in plants of the genus ''Aglaia'' (Meliaceae). These compounds are characterized by a cyclopenta[''b'']benzofuran skeleton. In 1982 King and colleagues discovered the first member of this family, rocaglamide, based on its antileukemic activity. Since then, about 50 other flavaglines have been characterized. These molecules display strong insecticidal, antifungal, anti-inflammatory, neuroprotective, cardioprotective and anticarcinogen, anticancer activities. In mouse models of cancer, flavaglines enhance the efficacy of chemotherapies and also alleviate the cardiac adverse effect of these chemotherapies. The challenge raised by their structural complexity has attracted the attention of some organic chemists. In 1990, Barry Trost presented an Enantiomer, enantioselective synthesis of rocaglamide in 18 steps and confirmed its absolute configuration. See also * FL3 (flavagline) *Rocaglamide *Silvestrol *Aglafoline Referenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rocaglamide
Rocaglamide is a natural product which belongs to a class of molecules called flavaglines. This compound was isolated in 1982 by King, Ming-Lu (金明儒) and colleagues based on its antileukemic activity. The name of Rocaglamide is named from two parts: Roc- and aglamide. Roc- means Republic of China(中華民國), the place in which this product isolated; aglamide indicates this product is isolated from Large-leaved Aglaia (Scientific name: Aglaia rimosa Rocaglamide was first synthesized by Barry Trost in 1990. Although other syntheses have been described since, Trost’s remains the only one to afford rocaglamide in an enantio-specific manner. See also * FL3 (flavagline) *Eukaryotic translation *eIF4A *Silvestrol Silvestrol is a natural product from the flavagline family, with a cyclopenta enzofuran core structure and an unusual dioxane ether side chain, which is found in the bark of trees from the genus ''Aglaia'', especially '' Aglaia silvestris'' and ' ... References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rocaglamide
Rocaglamide is a natural product which belongs to a class of molecules called flavaglines. This compound was isolated in 1982 by King, Ming-Lu (金明儒) and colleagues based on its antileukemic activity. The name of Rocaglamide is named from two parts: Roc- and aglamide. Roc- means Republic of China(中華民國), the place in which this product isolated; aglamide indicates this product is isolated from Large-leaved Aglaia (Scientific name: Aglaia rimosa Rocaglamide was first synthesized by Barry Trost in 1990. Although other syntheses have been described since, Trost’s remains the only one to afford rocaglamide in an enantio-specific manner. See also * FL3 (flavagline) *Eukaryotic translation *eIF4A *Silvestrol Silvestrol is a natural product from the flavagline family, with a cyclopenta enzofuran core structure and an unusual dioxane ether side chain, which is found in the bark of trees from the genus ''Aglaia'', especially '' Aglaia silvestris'' and ' ... References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aglaia
''Aglaia'' is a genus of 117 species of woody dioecious trees belonging to the Mahogany family (Meliaceae). These trees occur in the subtropical and tropical forests of Southeast Asia, Northern Australia and the Pacific. Some species are important timber trees; others have scented flowers, or medicinal properties (the edible fruits duku or langsat have now been placed in the genus '' Lansium''). Many have complex biological relationships with their dispersal agents. Phytochemistry Species in the genus ''Aglaia'' synthesize a unique class of highly bioactive chemical compounds known as flavaglines. Over 50 unique compounds of this class have been described so far, including rocaglamide, aglafoline, silvestrol, pannellin, episilvestrol, and ponapensin. They are known for their anti-cancer, anti-fungal, anti-inflammatory and insecticidal properties. Several of these compounds have been shown to be exceptional therapeutic agents for cancer chemotherapy, however further res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FL3 (flavagline)
FL3 is a synthetic flavagline that displays potent anticarcinogen, anticancer and cardioprotectant activities. This compound induces the death of cancer cells by an original mechanism that involves the apoptosis-inducing factor and caspase 12, suggesting that it may improve the efficacy of cancer chemotherapies. It was also shown that FL3 may enhance the efficacy of one of the most widely used anticarcinogen, anticancer agents, doxorubicin, and alleviate its main adverse effect, cardiac damage. References Diols Benzofuran ethers at the benzene ring Organobromides {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silvestrol
Silvestrol is a natural product from the flavagline family, with a cyclopenta enzofuran core structure and an unusual dioxane ether side chain, which is found in the bark of trees from the genus ''Aglaia'', especially '' Aglaia silvestris'' and ''Aglaia foveolata''. Bioactivity It acts as a potent and selective inhibitor of the RNA helicase enzyme eIF4A, and has both broad-spectrum antiviral activity against diseases such as Ebola and coronaviruses, and anti-cancer properties, which makes it of considerable interest in medical research. However, as it cannot be extracted from tree bark in commercial amounts and is prohibitively complex to produce synthetically, practical applications have focused more on structurally simplified analogues such as CR-31-B. See also * Rocaglamide Rocaglamide is a natural product which belongs to a class of molecules called flavaglines. This compound was isolated in 1982 by King, Ming-Lu (金明儒) and colleagues based on its antileukemic a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Meliaceae
Meliaceae, the mahogany family, is a flowering plant family of mostly trees and shrubs (and a few herbaceous plants, mangroves) in the order Sapindales. They are characterised by alternate, usually pinnate leaves without stipules, and by syncarpous, apparently bisexual (but actually mostly cryptically unisexual) flowers borne in panicles, cymes, spikes, or clusters. Most species are evergreen, but some are deciduous, either in the dry season or in winter. The family includes about 53 genera and about 600 known species, with a pantropical distribution; one genus (''Toona'') extends north into temperate China and south into southeast Australia, another (''Synoum'') into southeast Australia, and another (''Melia'') nearly as far north. They most commonly grow as understory trees in rainforests, but are also found in mangroves and arid regions. The fossil record of the family extends back into the Late Cretaceous. Uses Various species are used for vegetable oil, soap-making, ins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
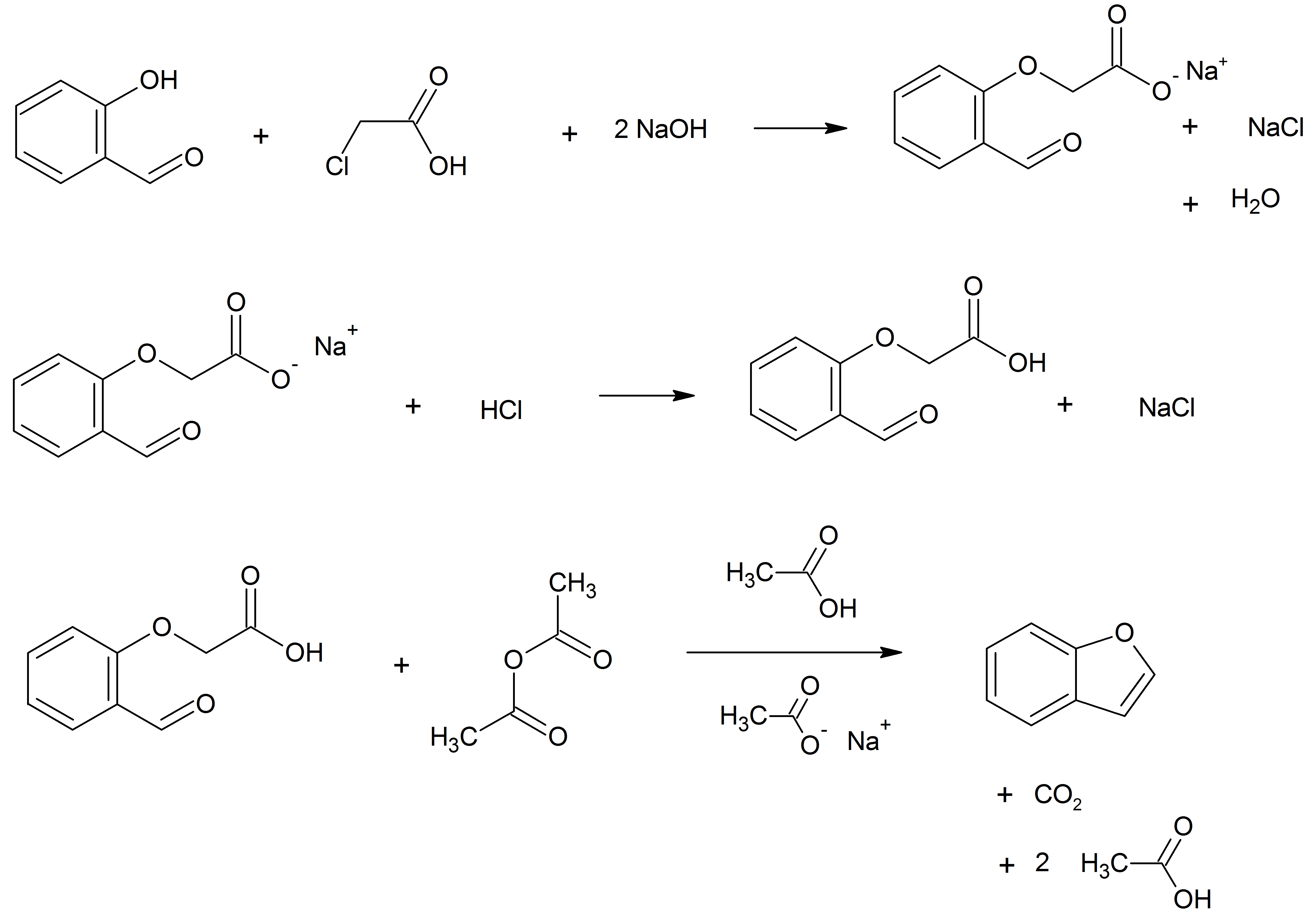
Benzofuran
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. *Perkin rearrangement, where a coumarin is reacted with a hydroxide: : * Diels–Alder reaction of nitro vinyl furans with various dienophiles: : Diels–Alder reaction yielding a substituted benzofuran, 450px * Cycloisomerization of alkyne ortho-substituted phenols: : Benzofurans via Cycloisomerization, 400px Related com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anticarcinogen
An anticarcinogen (also known as a carcinopreventive agent) is a substance that counteracts the effects of a carcinogen or inhibits the development of cancer. Anticarcinogens are different from anticarcinoma agents (also known as anticancer or anti-neoplastic agents) in that anticarcinoma agents are used to selectively destroy or inhibit cancer cells ''after'' cancer has developed. Interest in anticarcinogens is motivated primarily by the principle that it is preferable to prevent disease (preventive medicine) than to have to treat it ( rescue medicine). In theory, anticarcinogens may act via different mechanisms including enhancement of natural defences against cancer, deactivation of carcinogens, and blocking the mechanisms by which carcinogens act (such as free radical damage to DNA). Confirmation that a substance possesses anticarcinogenic activity requires extensive ''in vitro'', ''in vivo'', and clinical investigation. Health claims for anticarcinogens are regulated by v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barry Trost
Barry M. Trost (born June 13, 1941, in Philadelphia) is an American chemist who is the Job and Gertrud Tamaki Professor Emeritus in the School of Humanities and Sciences at Stanford University. The Tsuji-Trost reaction and the Trost ligand are named after him. He is prominent for advancing the concept of atom economy. Early life and education Trost was born in Philadelphia, Pennsylvania on June 13, 1941. He studied at the University of Pennsylvania and obtained his B.A. in 1962. He then attended the Massachusetts Institute of Technology for graduate school, where he worked with Herbert O. House on enolate anions, the Mannich reaction, and the Robinson annulation. Trost graduated with his Ph.D. in 1965. Independent career Trost moved to the University of Wisconsin–Madison to begin his independent career, and was promoted to Professor of Chemistry in 1969, and the Vilas Research Professor in 1982. In 1987, he moved to Stanford University as Professor of Chemistry, and w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical antipode – is one of two stereoisomers that are non-superposable onto their own mirror image. Enantiomers are much like one's right and left hands, when looking at the same face, they cannot be superposed onto each other. No amount of reorientation will allow the four unique groups on the chiral carbon (see Chirality (chemistry)) to line up exactly. The number of stereoisomers a molecule has can be determined by the number of chiral carbons it has. Stereoisomers include both enantiomers and diastereomers. Diastereomers, like enantiomers, share the same molecular formula and are non-superposable onto each other however, they are not mirror images of each other. A molecule with chirality rotates plane-polarized light. A mixture of equals a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |