|
Benzofuran
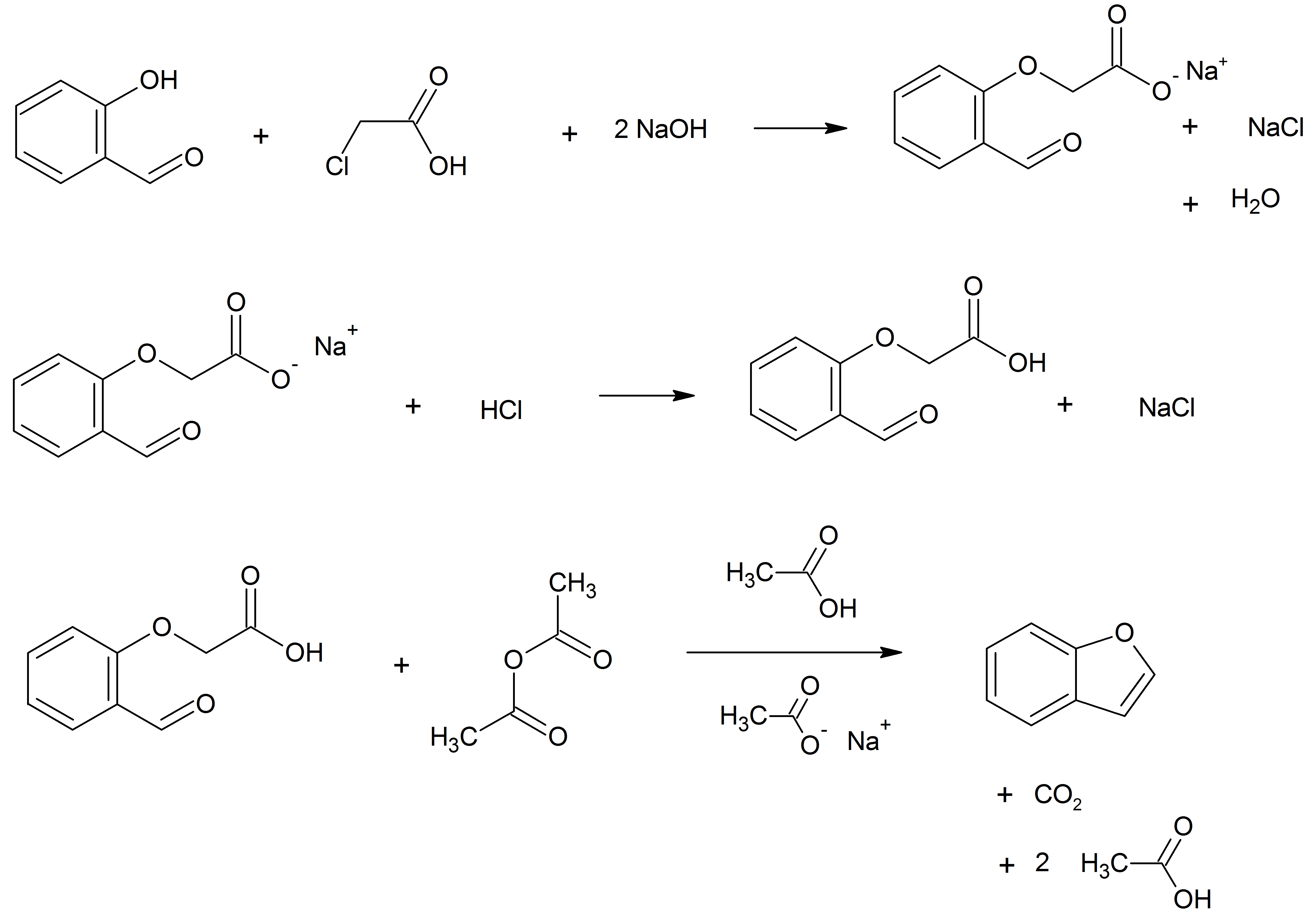
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. *Perkin rearrangement, where a coumarin is reacted with a hydroxide: : *Diels–Alder reaction of nitro vinyl furans with various dienophiles: : Diels–Alder reaction yielding a substituted benzofuran, 450px *Cycloisomerization of alkyne ortho-substituted phenols: : Benzofurans via Cycloisomerization, 400px Related compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Benzofuran
The substituted benzofurans are a class of chemical compounds based on the heterocyclyc and polycyclic compound benzofuran. Many medicines use the benzofuran core as a scaffold, but most commonly the term is used to refer to the simpler compounds in this class which include numerous psychoactive drugs, including stimulants, psychedelics and empathogens. In general, these compounds have a benzofuran core to which a 2-aminoethyl group is attached (at any position), and combined with a range of other substituents. Some psychoactive derivatives from this family have been sold under the name '' Benzofury''. List of substituted benzofurans The derivatives may be produced by substitutions at six locations of the benzofuran molecule, as well as saturation of the 2,3- double bond. The following table displays notable derivatives that have been reported: Legislation Substituted benzofurans saw widespread use as recreational drugs by being sold as research chemicals making them e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzofurans Via Cycloisomerization Of Alkynes
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethyl phenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. * Perkin rearrangement, where a coumarin is reacted with a hydroxide: : *Diels–Alder reaction of nitro vinyl furans with various dienophiles: : Diels–Alder reaction yielding a substituted benzofuran, 450px *Cycloisomerization of alkyne ortho-substituted phenols: : Benzofurans via Cycloisomerization, 400px ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzofuran
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. *Perkin rearrangement, where a coumarin is reacted with a hydroxide: : *Diels–Alder reaction of nitro vinyl furans with various dienophiles: : Diels–Alder reaction yielding a substituted benzofuran, 450px *Cycloisomerization of alkyne ortho-substituted phenols: : Benzofurans via Cycloisomerization, 400px Related compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salicylaldehyde
Salicylic aldehyde (2-hydroxybenzaldehyde) is the organic compound with the formula (C7 H6 O2) C6H4CHO-2-OH. Along with 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde. This colorless oily liquid has a bitter almond odor at higher concentration. Salicylaldehyde is a key precursor to a variety chelating agents, some of which are commercially important. Production Salicylaldehyde is prepared from phenol and chloroform by heating with sodium hydroxide or potassium hydroxide in a Reimer–Tiemann reaction: : Alternatively, it is produced by condensation of phenol or its derivatives with formaldehyde to give hydroxybenzyl alcohol, which is oxidized to the aldehyde. Salicylaldehydes in general may be prepared by other ortho-selective formylation reactions from the corresponding phenol, for instance by the Duff reaction, or by treatment with paraformaldehyde in the presence of magnesium chloride and a base. Natural ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perkin Rearrangement
In organic chemistry, the Perkin rearrangement (coumarin–benzofuran ring contraction) is a rearrangement reaction in which a 2-halocoumarin in the presence of hydroxide undergoes a ring contraction to form a benzofuran. The name reaction recognizes William Henry Perkin, who first reported it in 1870. Several proposals have been made for the reaction mechanism, all of which involve initial opening of the lactone to give a carboxylate and phenolate. : References {{organic-chem-stub Benzofurans Coumarins Name reactions Rearrangement reactions Ring contraction reactions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic Compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different chemical element, elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans. Furan is a colorless, flammable, highly volatile liquid with a boiling point close to room temperature. It is soluble in common organic solvents, including alcohol, ether, and acetone, and is slightly soluble in water. Its odor is "strong, ethereal; chloroform-like". It is toxic and may be carcinogenic in humans. Furan is used as a starting point for other speciality chemicals. History The name "furan" comes from the Latin ''furfur'', which means bran. (Furfural is produced from bran.) The first furan derivative to be described was 2-furoic acid, by Carl Wilhelm Scheele in 1780. Another important derivative, furfural, was reported by Johann Wolfgang Döbereiner in 1831 and characterised nine years later by John Stenhouse. Furan itself was first prepared by Heinrich Limp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psoralen
Psoralen (also called psoralene) is the parent compound in a family of naturally occurring organic compounds known as the linear furanocoumarins. It is structurally related to coumarin by the addition of a fused furan ring, and may be considered as a derivative of umbelliferone. Psoralen occurs naturally in the seeds of '' Psoralea corylifolia'', as well as in the common fig, celery, parsley, West Indian satinwood, and in all citrus fruits. It is widely used in PUVA (psoralen + UVA) treatment for psoriasis, eczema, vitiligo, and cutaneous T-cell lymphoma; these applications are typically through the use of medications such as Methoxsalen. Many furanocoumarins are extremely toxic to fish, and some are deposited in streams in Indonesia to catch fish. Uses Psoralen is a mutagen, and is used for this purpose in molecular biology research. Psoralen intercalates into DNA and on exposure to ultraviolet (UVA) radiation can form monoadducts and covalent interstrand cross-links ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. The corresponding substituent is called indolyl. Indole undergoes electrophilic substitution, mainly at position 3 (see diagr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroacetic Acid
Chloroacetic acid, industrially known as monochloroacetic acid (MCA), is the organochlorine compound with the formula ClCH2CO2H. This carboxylic acid is a useful building block in organic synthesis. It is a colorless solid. Related compounds are dichloroacetic acid and trichloroacetic acid. Production Chloroacetic acid was first prepared (in impure form) by the French chemist Félix LeBlanc (1813–1886) in 1843 by chlorinating acetic acid in the presence of sunlight, and in 1857 (in pure form) by the German chemist Reinhold Hoffmann (1831–1919) by refluxing glacial acetic acid in the presence of chlorine and sunlight, and then by the French chemist Charles Adolphe Wurtz by hydrolysis of chloroacetyl chloride (ClCH2COCl), also in 1857. Chloroacetic acid is prepared industrially by two routes. The predominant method involves chlorination of acetic acid, with acetic anhydride as a catalyst: : + → + This route suffers from the production of dichloroacetic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Furans
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans. Furan is a colorless, flammable, highly volatile liquid with a boiling point close to room temperature. It is soluble in common organic solvents, including alcohol, ether, and acetone, and is slightly soluble in water. Its odor is "strong, ethereal; chloroform-like". It is toxic and may be carcinogenic in humans. Furan is used as a starting point for other speciality chemicals. History The name "furan" comes from the Latin ''furfur'', which means bran. (Furfural is produced from bran.) The first furan derivative to be described was 2-furoic acid, by Carl Wilhelm Scheele in 1780. Another important derivative, furfural, was reported by Johann Wolfgang Döbereiner in 1831 and characterised nine years later by John Stenhouse. Furan itself was first prepared by Heinrich Limp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |