|
Ethoxylate
Ethoxylation is a chemical reaction in which ethylene oxide adds to a substrate. It is the most widely practiced alkoxylation, which involves the addition of epoxides to substrates. In the usual application, alcohols and phenols are converted into R(OC2H4)nOH where n ranges from 1 to 10. Such compounds are called alcohol ethoxylates. Alcohol ethoxylates are often converted to related species called ethoxysulfates. Alcohol ethoxylates and ethoxysulfates are surfactants, used widely in cosmetic and other commercial products. The process is of great industrial significance with more than 2,000,000 metric tons of various ethoxylates produced worldwide in 1994. Production The process was developed at the Ludwigshafen laboratories of IG Farben by Conrad Schöller and during the 1930s. Alcohol ethoxylates Industrial ethoxylation is primarily performed upon fatty alcohols in order to generate fatty alcohol ethoxylates (FAE's), which are a common form of nonionic surfactant (e.g. octa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonionic Surfactant
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, or dispersants. The word "surfactant" is a blend of ''surface-active agent'', coined . Agents that increase surface tension are "surface active" in the literal sense but are not called surfactants as their effect is opposite to the common meaning. A common example of surface tension increase is salting out: by adding an inorganic salt to an aqueous solution of a weakly polar substance, the substance will precipitate. The substance may itself be a surfactant – this is one of the reasons why many surfactants are ineffective in sea water. Composition and structure Surfactants are usually organic compounds that are amphiphilic, meaning each molecule contains both a hydrophilic "water-seeking" group (the ''head''), and a hy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anionic Surfactant
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, or dispersants. The word "surfactant" is a blend of ''surface-active agent'', coined . Agents that increase surface tension are "surface active" in the literal sense but are not called surfactants as their effect is opposite to the common meaning. A common example of surface tension increase is salting out: by adding an inorganic salt to an aqueous solution of a weakly polar substance, the substance will precipitate. The substance may itself be a surfactant – this is one of the reasons why many surfactants are ineffective in sea water. Composition and structure Surfactants are usually organic compounds that are amphiphilic, meaning each molecule contains both a hydrophilic "water-seeking" group (the ''head''), and a hydrop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surfactant
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, or dispersants. The word "surfactant" is a blend of ''surface-active agent'', coined . Agents that increase surface tension are "surface active" in the literal sense but are not called surfactants as their effect is opposite to the common meaning. A common example of surface tension increase is salting out: by adding an inorganic salt to an aqueous solution of a weakly polar substance, the substance will precipitate. The substance may itself be a surfactant – this is one of the reasons why many surfactants are ineffective in sea water. Composition and structure Surfactants are usually organic compounds that are amphiphilic, meaning each molecule contains both a hydrophilic "water-seeking" group (the ''head''), and a hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Oxide
Ethylene oxide is an organic compound with the chemical formula, formula . It is a cyclic ether and the simplest epoxide: a three-membered Ring (chemistry), ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of silver catalyst. The reactivity that is responsible for many of ethylene oxide's hazards also makes it useful. Although too dangerous for direct household use and generally unfamiliar to consumers, ethylene oxide is used for making many consumer products as well as non-consumer chemicals and intermediates. These products include detergents, thickeners, solvents, plastics, and various organic chemicals such as ethylene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant pressure, which is conveniently provided by the large ambient atmosphere. The pressure–volume term expresses the work required to establish the system's physical dimensions, i.e. to make room for it by displacing its surroundings. The pressure-volume term is very small for solids and liquids at common conditions, and fairly small for gases. Therefore, enthalpy is a stand-in for energy in chemical systems; bond, lattice, solvation and other "energies" in chemistry are actually enthalpy differences. As a state function, enthalpy depends only on the final configuration of internal energy, pressure, and volume, not on the path taken to achieve it. In the International System of Units (SI), the unit of measurement for enthalpy is the joule. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
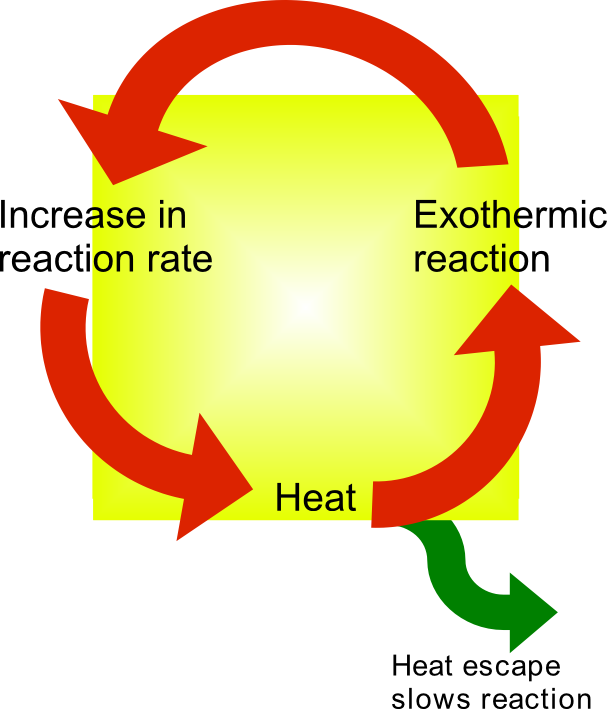
Thermal Runaway
Thermal runaway describes a process that is accelerated by increased temperature, in turn releasing energy that further increases temperature. Thermal runaway occurs in situations where an increase in temperature changes the conditions in a way that causes a further increase in temperature, often leading to a destructive result. It is a kind of uncontrolled positive feedback. In chemistry (and chemical engineering), thermal runaway is associated with strongly exothermic reactions that are accelerated by temperature rise. In electrical engineering, thermal runaway is typically associated with increased current flow and power dissipation. Thermal runaway can occur in civil engineering, notably when the heat released by large amounts of curing concrete is not controlled. In astrophysics, runaway nuclear fusion reactions in stars can lead to nova and several types of supernova explosions, and also occur as a less dramatic event in the normal evolution of solar-mass stars, the " he ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primary Alcohols
A primary alcohol is an alcohol in which the hydroxy group In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ... is bonded to a primary carbon atom. It can also be defined as a molecule containing a “–CH2OH” group. In contrast, a secondary alcohol has a formula “–CHROH” and a tertiary alcohol has a formula “–CR2OH”, where “R” indicates a carbon-containing group. Examples of primary alcohols include ethanol and n-Butanol, 1-butanol. Methanol is also generally regarded as a primary alcohol, including the 1911 edition of the Encyclopædia Britannica,. See also * Alcohol (chemistry), Alcohol (especially Nomenclature section for discussion on Secondary and Tertiary alcohols.) * Oxidation of primary alcohols to carboxylic acids References Primary alcohols, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Repeat Unit
In polymer chemistry, a repeat unit or repeating unit (or mer) is a part of a polymer whose repetition would produce the complete polymer chain (except for the end-groups) by linking the repeat units together successively along the chain, like the beads of a necklace. A repeat unit is sometimes called a mer (or mer unit). "Mer" originates from the Greek word ''meros'', which means "a part". The word polymer derives its meaning from this, which means "many mers". A repeat unit (mer) is not to be confused with the term monomer, which refers to the small molecule from which a polymer is synthesized.Callister, William D. (2007). ''Materials science and engineering : an introduction'' (7th ed.) New York : John Wiley & Sons. One of the simplest repeat units is that of the addition polymer polyvinyl chloride, - H2-CHClsub>n-, whose repeat unit is - H2-CHCl. In this case the repeat unit has the same atoms as the monomer vinyl chloride CH2=CHCl. When the polymer is formed, the C=C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Narrow-range Ethoxylate
Narrow-range ethoxylates (NREs) in chemistry are fatty alcohol polyglycol ethers with a narrow homolog distribution and are known nonionic surfactants. They can be produced industrially, for example, by the addition of ethylene oxide onto fatty alcohols in the presence of suitable catalysts (layer compounds which have been calcined or hydrophobized with fatty acids).Reviews on this subject are presented, for example, by M. Cox in '' J. Am. Oil Chem. Soc.'' 67, 599 (1990) and by H. Hensen et al. in Seifen-Ole-Fette-Wachse, 117, 592 (1991). This process can also be carried out on a variety of other hydrophobes and using different alkoxylating compounds (e.g., propylene oxide and butylene oxide) by modifying the catalyst properties. Example An ethoxylation reaction proceeds under an inert atmosphere with an amount of heat depending on the starting material. The reaction proceeds via the epoxide (in this case ethylene oxide) ring opening and activation of the nucleophile, ring, or comb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (chemistry), products, which usually have properties different from the reactants. Reactions often consist of a sequence o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High Production Volume Chemicals Programme
High production volume chemicals (HPV chemicals) are produced or imported into the United States in quantities of 1 million pounds or 500 tons per year. In OECD countries, HPV chemicals are defined as being produced at levels greater than 1,000 metric tons per producer/importer per year in at least one member country/region. A list of HPV chemicals serves as an overall priority list, from which chemicals are selected to gather data for a screening information dataset (SIDS), for testing and for initial hazard assessment. History OECD countries including EU In 1987, member countries of the Organisation for Economic Co-operation and Development decided to investigate existing chemicals. In 1991, they agreed to begin by focusing on High production volume (HPV) chemicals, where production volume was used as a surrogate for data on occupational, consumer, and environmental exposure. Each country agreed to "sponsor" the assessment of a proportion of the HPV chemicals. Countries also a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |