|
Disiamylborane
Disiamylborane (bis(1,2-dimethylpropyl)borane, Sia2BH) is an organoborane used in organic synthesis. It is used for hydroboration–oxidation reactions of terminal alkynes, giving aldehydes via anti-Markovnikov hydration followed by tautomerization. Disiamylborane is relatively selective for terminal alkynes and alkenes vs internal alkynes and alkenes.{{OrgSynth, author=Eric J. Leopold , title=Selective Hydroboration of a 1,3,7-Triene: Homogeraniol, volume= 64, year=1986, page=164, doi=10.15227/orgsyn.064.0164 Disiamylborane is prepared by hydroboration of trimethylethylene with diborane. The reaction stops at the secondary borane due to steric hindrance. Related reagents * 9-Borabicyclo .3.1onane (9-BBN). * Thexylborane ((1,1,2-trimethylpropyl)borane, ThxBH2), a primary borane obtained by hydroboration of tetramethylethylene Tetramethylethylene is a hydrocarbon with the formula Me2C=CMe2 (Me = methyl). A colorless liquid, it is the simplest tetrasubstituted alkene. Synthes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroboration–oxidation Reaction
Hydroboration–oxidation reaction is a two-step hydration reaction that converts an alkene into an alcohol. The process results in the syn addition of a hydrogen and a hydroxyl group where the double bond had been. Hydroboration–oxidation is an anti-Markovnikov reaction, with the hydroxyl group attaching to the less-substituted carbon. The reaction thus provides a more stereospecific and complementary regiochemical alternative to other hydration reactions such as acid-catalyzed addition and the oxymercuration–reduction process. The reaction was first reported by Herbert C. Brown in the late 1950s and it was recognized in his receiving the Nobel Prize in Chemistry in 1979. The general form of the reaction is as follows: Tetrahydrofuran (THF) is the archetypal solvent used for hydroboration. Mechanism and scope Hydroboration step In the first step, borane (BH3) adds to the double bond, transferring one of the hydrogen atoms to the carbon adjacent to the one that becom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organoborane
Organoborane or organoboron compounds are chemical compounds of boron and carbon that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds. Organoboron compounds are important reagents in organic chemistry enabling many chemical transformations, the most important one called hydroboration. Reactions of organoborates and boranes involve the transfer of a nucleophilic group attached to boron to an electrophilic center either inter- or intramolecularly. α,β-Unsaturated borates, as well as borates with a leaving group at the α position, are highly susceptible to intramolecular 1,2-migration of a group from boron to the electrophilic α position. Oxidation or protonolysis of the resulting organoboranes may generate a variety of organic products, including alcohols, carbonyl compounds, alkenes, and halides. Properties of the B-C bond The C-B bond has low polarity (the difference in electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
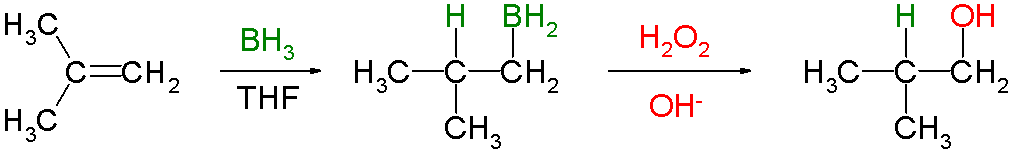
Hydroboration
In organic chemistry, hydroboration refers to the addition of a hydrogen-boron bond to certain double and triple bonds involving carbon (, , , and ). This chemical reaction is useful in the organic synthesis of organic compounds. Hydroboration produces organoborane compounds that react with a variety of reagents to produce useful compounds, such as alcohols, amines, or alkyl halides. The most widely known reaction of the organoboranes is oxidation to produce alcohols typically by hydrogen peroxide. This type of reaction has promoted research on hydroboration because of its mild condition and a wide scope of tolerated alkenes. Another research subtheme is metal-catalysed hydroboration. The development of this technology and the underlying concepts were recognized by the Nobel Prize in Chemistry to Herbert C. Brown. He shared the prize with Georg Wittig in 1979 for his pioneering research on organoboranes as important synthetic intermediates. Addition of a H-B bond to C-C doubl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes (134 pm) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contribute ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres. Structure and bonding Aldehydes feature a carbon center that is connected by a double bond to oxygen and a single bond to hydrogen and single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The C=O bond length is about 120-122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile aldehydes have pungent odors. Al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Markovnikov's Rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule describes the outcome of some addition reactions. The rule was formulated by Russian chemist Vladimir Markovnikov in 1870. Explanation The rule states that with the addition of a protic acid HX or other polar reagent to an asymmetric alkene, the acid hydrogen (H) or electropositive part gets attached to the carbon with more hydrogen substituents, and the halide (X) group or electronegative part gets attached to the carbon with more alkyl substituents. This is in contrast to Markovnikov's original definition, in which the rule is stated that the X component is added to the carbon with the fewest hydrogen atoms while the hydrogen atom is added to the carbon with the greatest number of hydrogen atoms. The same is true when an alkene reacts with water in an addition reaction to form an alcohol which involve formation of carbocations. The hydroxyl group (OH) bonds to the carbon that has the greater number of carbon– ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tautomer
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert. The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydrogen atom within the compound. The phenomenon of tautomerization is called tautomerism, also called desmotropism. Tautomerism is for example relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life. Care should be taken not to confuse tautomers with depictions of "contributing structures" in chemical resonance. Tautomers are distinct chemical species that can be distinguished by their differing atomic connectivities, molecular geometries, and physicochemical and spectroscopic properties, whereas resonance forms are merely alternative Lewis structure (valence bond theory) depictions of a single chemical species, whose true structure is best described as the "average" of the idealized, hypothe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylethylene
2-Methyl-2-butene, 2m2b, 2-methylbut-2-ene, also beta-isoamylene is an alkene hydrocarbon with the molecular formula C5H10. Used as a free radical scavenger in trichloromethane (chloroform) and dichloromethane (methylene chloride). John Snow, the English physician, experimented with it in the 1840s as an anesthetic, but stopped using it for unknown reasons. See also *Pentene Pentenes are alkenes with the chemical formula . Each contains one double bond within its molecular structure. Six different compounds are in this class, differing from each other by whether the carbon atoms are attached linearly or in a branched ... References Hydrocarbons Alkenes {{hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diborane
Diborane(6), generally known as diborane, is the chemical compound with the formula B2H6. It is a toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Diborane is a key boron compound with a variety of applications. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents. Structure and bonding The structure of diborane has D2h symmetry. Four hydrides are terminal, while two bridge between the boron centers. The lengths of the B–Hbridge bonds and the B–Hterminal bonds are 1.33 and 1.19 Å respectively. This difference in bond lengths reflects the difference in their strengths, the B–Hbridge bonds being relatively weaker. The weakness of the B–Hbridge compared to B–Hterminal bonds is indicated by their vibrational signatures in the infrared spectrum, being ≈2100 and 2500 cm−1 respectively. The model determined by molecular orbital theory describes the bonds between boron and the termina ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thexylborane
Thexylborane is a borane with the formula e2CHCMe2BH2sub>2 (Me = methyl). The name derives from "''t''-hexylborane" (although the group is not the standard ''tert''-hexyl group), and the formula is often abbreviated ThxBH2. A colorless liquid, it is a rare, easily accessed monoalkylborane. It is produced by the hydroboration of tetramethylethylene: :B2H6 + 2 Me2C=CMe2 → e2CHCMe2BH2sub>2 Reactions Thexylborane is generated ''in situ ''In situ'' (; often not italicized in English) is a Latin phrase that translates literally to "on site" or "in position." It can mean "locally", "on site", "on the premises", or "in place" to describe where an event takes place and is used in ...''. In solution, it isomerizes over the course several days to the 2,3-dimethyl-1-butyl derivative, shown as the monomer here: :Me2CHCMe2BH2 → Me2CHCH(Me)CH2BH2 Thexylborane allows the synthesis of ketones by coupling a pair of alkenes with carbon monoxide, which serves as a carbonyl linc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |