|
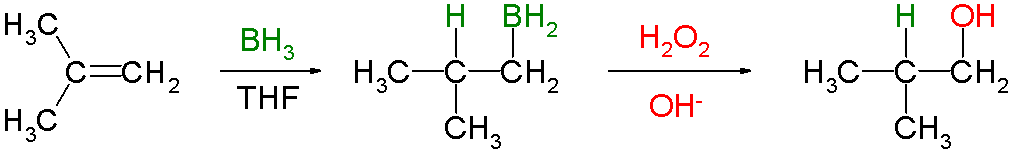
Hydroboration–oxidation Reaction
Hydroboration–oxidation reaction is a two-step hydration reaction that converts an alkene into an Alcohol (chemistry), alcohol. The process results in the Syn and anti addition, syn addition of a hydrogen and a hydroxyl group where the double bond had been. Hydroboration–oxidation is an Markovnikov's rule, anti-Markovnikov reaction, with the hydroxyl group attaching to the less-substituted carbon. The reaction thus provides a more stereospecific and complementary regiochemistry, regiochemical alternative to other hydration reactions such as Acid catalysis, acid-catalyzed addition and the Oxymercuration reaction, oxymercuration–reduction process. The reaction was first reported by Herbert C. Brown in the late 1950s and it was recognized in his receiving the Nobel Prize in Chemistry in 1979. The general form of the reaction is as follows: Tetrahydrofuran (THF) is the archetypal solvent used for hydroboration. Mechanism and scope Hydroboration step In the first step, borane ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydration Reaction
In chemistry, a hydration reaction is a chemical reaction in which a substance combines with water. In organic chemistry, water is added to an unsaturated substrate, which is usually an alkene or an alkyne. This type of reaction is employed industrially to produce ethanol, isopropanol, and butan-2-ol.. Organic chemistry Epoxides to glycol Several million tons of ethylene glycol are produced annually by the hydration of oxirane, a cyclic compound also known as ethylene oxide: : C2H4O + H2O → HO–CH2CH2–OH Acid catalysts are typically used. Alkenes For the hydration of alkenes, the general chemical equation of the reaction is the following: :RRC=CH2 + H2O → RRC(OH)-CH3 A hydroxyl group (OH−) attaches to one carbon of the double bond, and a proton (H+) adds to the other. The reaction is highly exothermic. In the first step, the alkene acts as a nucleophile and attacks the proton, following Markovnikov's rule. In the second step an H2O molecule bonds to the other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the '' boron group'' it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride. Boron is synthesized entirely by cosmic ray spallation and supernovae and not by stellar nucleosynthesis, so it is a low-abundance element in the Solar System and in the Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. It is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known deposits are in Turkey, the largest producer of boron minerals. Elemental boron is a meta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Walden Inversion
Walden inversion is the inversion of a stereogenic center in a chiral molecule in a chemical reaction. Since a molecule can form two enantiomers around a stereogenic center, the Walden inversion converts the configuration of the molecule from one enantiomeric form to the other. For example, in an SN2 reaction, Walden inversion occurs at a tetrahedral carbon atom. It can be visualized by imagining an umbrella turned inside-out in a gale. In the Walden inversion, the backside attack by the nucleophile in an SN2 reaction gives rise to a product whose configuration is opposite to the reactant. Therefore, during SN2 reaction, 100% inversion of product takes place. This is known as Walden inversion. It was first observed by chemist Paul Walden in 1896. He was able to convert one enantiomer of a chemical compound into the other enantiomer and back again in a so-called Walden cycle which went like this: (+) chlorosuccinic acid (1 in the illustration) was converted to (+) malic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroperoxide
Hydroperoxides or peroxols are compounds containing the hydroperoxide functional group (ROOH). If the R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. Organic hydroperoxides can either intentionally or unintentionally initiate explosive polymerisation in materials with unsaturated chemical bonds. Properties The O−O bond length in peroxides is about 1.45 Å, and the R−O−O angles (R = H, C) are about 110° (water-like). Characteristically, the C−O−O−H dihedral angles are about 120°. The O−O bond is relatively weak, with a bond dissociation energy of , less than half the strengths of C−C, C−H, and C−O bonds. Hydroperoxides are typically more volatile than the corresponding alcohols: * ''tert''-BuOOH (b.p. 36°C) vs ''tert''-BuOH (b.p. 82-83°C) * CH3OOH (b.p. 46°C) vs CH3OH (b.p. 65°C * cumene hydroperoxide (b.p. 153°C) vs cumyl alcohol (b.p. 202°C) Misc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disiamylborane
Disiamylborane (bis(1,2-dimethylpropyl)borane, Sia2BH) is an organoborane used in organic synthesis. It is used for hydroboration–oxidation reactions of terminal alkynes, giving aldehydes via anti-Markovnikov hydration followed by tautomerization. Disiamylborane is relatively selective for terminal alkynes and alkenes vs internal alkynes and alkenes.{{OrgSynth, author=Eric J. Leopold , title=Selective Hydroboration of a 1,3,7-Triene: Homogeraniol, volume= 64, year=1986, page=164, doi=10.15227/orgsyn.064.0164 Disiamylborane is prepared by hydroboration of trimethylethylene with diborane. The reaction stops at the secondary borane due to steric hindrance. Related reagents * 9-Borabicyclo .3.1onane (9-BBN). * Thexylborane ((1,1,2-trimethylpropyl)borane, ThxBH2), a primary borane obtained by hydroboration of tetramethylethylene Tetramethylethylene is a hydrocarbon with the formula Me2C=CMe2 (Me = methyl). A colorless liquid, it is the simplest tetrasubstituted alkene. Synthesi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catecholborane
Catecholborane (abbreviated HBcat) is an organoboron compound that is useful in organic synthesis. This colourless liquid is a derivative of catechol and a borane, having the formula C6H4O2BH. Synthesis and structure Traditionally catecholborane is produced by treating catechol with borane (BH3) in a cooled solution of THF. However, this method results in a loss of 2 mole equivalents of the hydride. Nöth and Männig described the reaction of alkali-metal boron hydride (LiBH4, NaBH4, of KBH4) with tris(catecholato)bisborane in an ethereal solvent such as diethyl ether. In 2001, Herbert Brown and coworkers prepared catecholborane by treatment of tri-''o''-phenylene bis-borate with diborane. Unlike borane itself or alkylboranes, catechol borane exists as a monomer. This behavior is a consequence of the electronic influence of the aryloxy groups that diminish the Lewis acidity of the boron centre. Pinacolborane adopts a similar structure. Reactions Catecholborane is less reactiv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Syn Addition
In organic chemistry, syn- and anti-addition are different ways in which substituent molecules can be added to an alkene or alkyne. The concepts of syn and anti addition are used to characterize the different reactions of organic chemistry by reflecting the stereochemistry of the products in a reaction. The type of addition that occurs depends on multiple different factors of a reaction, and is defined by the final orientation of the substituents on the parent molecule. Syn and anti addition are related to the Markovnikov's rule for the orientation of a reaction, which refers to the bonding preference of different substituents for different carbons on an alkene or alkyne. In order for a reaction to follow Markovnikov's rule, the intermediate carbocation of the mechanism of a reaction must be on the more substituted carbon, allowing the substituent to bond to the more stable carbocation and the more substituted carbon. Syn addition is the addition of two substituents to the same s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Markovnikov Addition
In organic chemistry, Markovnikov's rule or Markownikoff's rule describes the outcome of some addition reactions. The rule was formulated by Russian chemist Vladimir Markovnikov in 1870. Explanation The rule states that with the addition of a protic acid HX or other polar reagent to an asymmetric alkene, the acid hydrogen (H) or electropositive part gets attached to the carbon with more hydrogen substituents, and the halide (X) group or electronegative part gets attached to the carbon with more alkyl substituents. This is in contrast to Markovnikov's original definition, in which the rule is stated that the X component is added to the carbon with the fewest hydrogen atoms while the hydrogen atom is added to the carbon with the greatest number of hydrogen atoms. The same is true when an alkene reacts with water in an addition reaction to form an alcohol which involve formation of carbocations. The hydroxyl group (OH) bonds to the carbon that has the greater number of carbon–ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-Hexanol
1-Hexanol (IUPAC name hexan-1-ol) is an organic alcohol with a six-carbon chain and a condensed structural formula of CH3(CH2)5OH. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. Two additional straight chain isomers of 1-hexanol, 2-hexanol and 3-hexanol, exist, both of which differing by the location of the hydroxyl group. Many isomeric alcohols have the formula C6H13OH. It is used in the perfume industry. Preparation Hexanol is produced industrially by the oligomerization of ethylene using triethylaluminium followed by oxidation of the alkylaluminium products.. An idealized synthesis is shown: :Al(C2H5)3 + 6C2H4 → Al(C6H13)3 :Al(C6H13)3 + O2 + 3H2O → 3HOC6H13 + Al(OH)3 The process generates a range of oligomers that are separated by distillation. Alternative methods Another method of preparation entails hydroformylation of 1-pentene followed by hydrogenation of the resulting aldehydes. This method is practiced in in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |