|
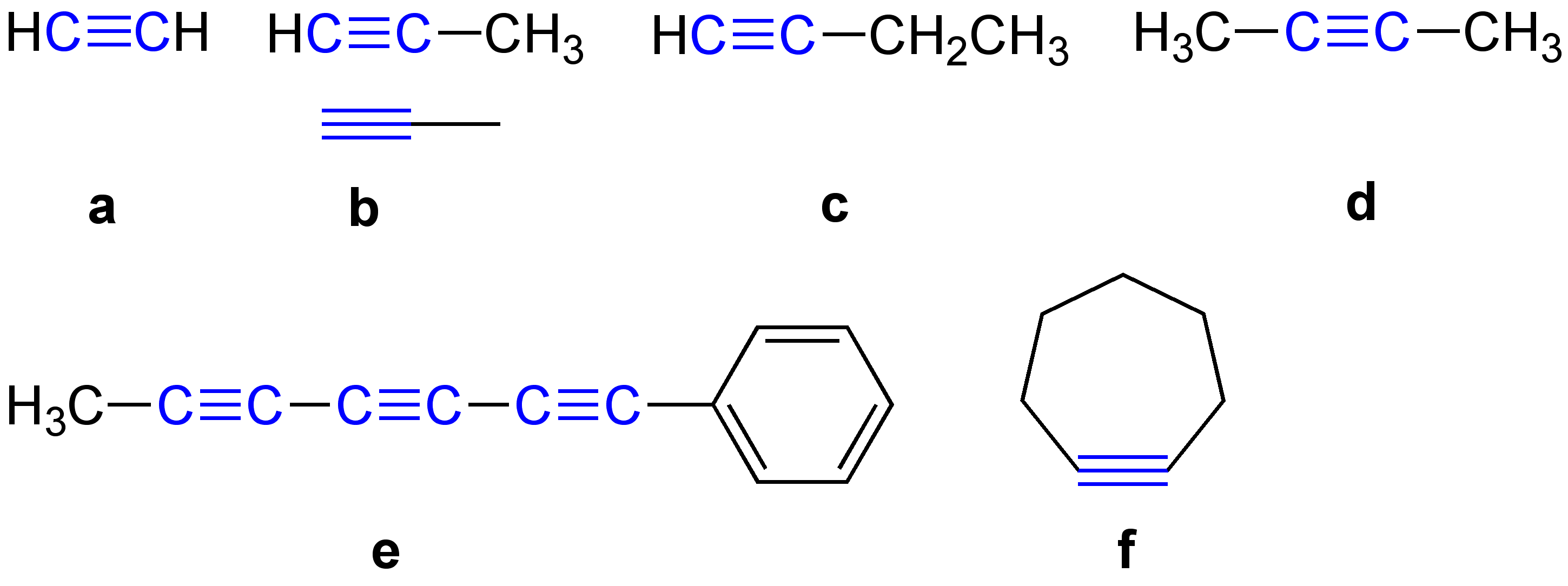
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes (134 pm) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contribute ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne General Formulae V
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes (134 pm) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contributes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC Nomenclature Of Organic Chemistry
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the ''Nomenclature of Organic Chemistry'' (informally called the Blue Book). Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry. To avoid long and tedious names in normal communication, the official IUPAC naming recommendations are not always followed in practice, except when it is necessary to give an unambiguous and absolute definition to a compound. IUPAC names can sometimes be simpler than older names, as with ethanol, instead of ethyl alcohol. For relatively simple molecules they can be more easily understood than non-systematic names, which must be learnt or looked over. However, the common or trivial name is often substantially ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylene
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities such as divinyl sulfide and phosphine.Compressed Gas Association (1995Material Safety and Data Sheet – Acetylene As an alkyne, acetylene is unsaturated because its two carbon atoms are bonded together in a triple bond. The carbon–carbon triple bond places all four atoms in the same straight line, with CCH bond angles of 180°. Discovery Acetylene was discovered in 1836 by Edmund Davy, who identified it as a "new carburet of hydrogen". It was an accidental discovery while attempting to isolate potassium metal. By heating potassium carbonate with carbon at very high temperatures, he produced a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. Fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylacetylene
Propyne (methylacetylene) is an alkyne with the chemical formula . It is a component of MAPD gas—along with its isomer propadiene (allene), which was commonly used in gas welding. Unlike acetylene, propyne can be safely condensed.Peter Pässler, Werner Hefner, Klaus Buckl, Helmut Meinass, Andreas Meiswinkel, Hans-Jürgen Wernicke, Günter Ebersberg, Richard Müller, Jürgen Bässler, Hartmut Behringer, Dieter Mayer, "Acetylene" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, Weinheim 2007 (). Production and equilibrium with propadiene Propyne exists in equilibrium with propadiene, the mixture of propyne and propadiene being called MAPD: :H3CC#CH H2C=C=CH2 The coefficient of equilibrium ''K''eq is 0.22 at 270 °C or 0.1 at 5 °C. MAPD is produced as a side product, often an undesirable one, by cracking propane to produce propene, an important feedstock in the chemical industry. MAPD interferes with the catalytic polymerization of propene. Laborato ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propyne
Propyne (methylacetylene) is an alkyne with the chemical formula . It is a component of MAPD gas—along with its isomer propadiene (allene), which was commonly used in gas welding. Unlike acetylene, propyne can be safely condensed.Peter Pässler, Werner Hefner, Klaus Buckl, Helmut Meinass, Andreas Meiswinkel, Hans-Jürgen Wernicke, Günter Ebersberg, Richard Müller, Jürgen Bässler, Hartmut Behringer, Dieter Mayer, "Acetylene" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, Weinheim 2007 (). Production and equilibrium with propadiene Propyne exists in equilibrium with propadiene, the mixture of propyne and propadiene being called MAPD: :H3CC#CH H2C=C=CH2 The coefficient of equilibrium ''K''eq is 0.22 at 270 °C or 0.1 at 5 °C. MAPD is produced as a side product, often an undesirable one, by cracking propane to produce propene, an important feedstock in the chemical industry. MAPD interferes with the catalytic polymerization of propene. Laborato ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyne
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities such as divinyl sulfide and phosphine.Compressed Gas Association (1995Material Safety and Data Sheet – Acetylene As an alkyne, acetylene is unsaturated because its two carbon atoms are bonded together in a triple bond. The carbon–carbon triple bond places all four atoms in the same straight line, with CCH bond angles of 180°. Discovery Acetylene was discovered in 1836 by Edmund Davy, who identified it as a "new carburet of hydrogen". It was an accidental discovery while attempting to isolate potassium metal. By heating potassium carbonate with carbon at very high temperatures, he produced a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylene
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities such as divinyl sulfide and phosphine.Compressed Gas Association (1995Material Safety and Data Sheet – Acetylene As an alkyne, acetylene is unsaturated because its two carbon atoms are bonded together in a triple bond. The carbon–carbon triple bond places all four atoms in the same straight line, with CCH bond angles of 180°. Discovery Acetylene was discovered in 1836 by Edmund Davy, who identified it as a "new carburet of hydrogen". It was an accidental discovery while attempting to isolate potassium metal. By heating potassium carbonate with carbon at very high temperatures, he produced a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-Butyne
1-Butyne is an organic compound with the chemical formula HC2CH2CH3. It is a colorless combustable gas. 1-Butyne participates in reactions typical for terminal alkynes, such as alkyne metathesis, hydrogenation, condensation with formaldehyde. Based on its heat of combustion, it is slightly less stable than its isomer 2-butyne. See also * 2-Butyne * Butadiene * Cyclobutene Cyclobutene is a cycloalkene. It is of interest in research but currently has no practical applications. It is a colorless easily condensed gas. A modern synthesis involves the 2-step dehydration of cyclobutanol. The compound was first prepare ... References External links NIST Chemistry WebBook page for 1-butyne Alkynes {{hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or exemplified by the odors of gasoline and lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to the naturally occurring petroleum, natural gas and coal, and to their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are carbon di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphenylacetylene
Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of two phenyl groups attached to a C2 unit. A colorless solid, it is used as a building block in organic synthesis and as a ligand in organometallic chemistry. Preparation and structure In one preparation for this compound, benzil is condensed with hydrazine to give the bis(hydrazone), which is oxidized with mercury(II) oxide. Alternatively stilbene is brominated, and the resulting dibromodiphenylethane is subjected to dehydrohalogenation, Yet another method starts involves the coupling iodobenzene and the copper salt of phenylacetylene in the Castro-Stephens coupling. Diphenylacetylene is a planar molecule. The central C≡C distance is 119.8 picometers. Derivatives Reaction of diphenylacetylene with tetraphenylcyclopentadienone results in the formation of hexaphenylbenzene in a Diels–Alder reaction. Reaction of Ph2C2 with benzal chloride in the presence of potassium ''t''-butoxide affords t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |