|
Cyclophilin D
Peptidylprolyl isomerase D (cyclophilin D), also known as PPID, is an enzyme which in humans is encoded by the ''PPID'' gene on chromosome 4. As a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family, this protein catalyzes the cis-trans isomerization of proline imidic peptide bonds, which allows it to facilitate folding or repair of proteins. In addition, PPID participates in many biological processes, including mitochondrial metabolism, apoptosis, redox, and inflammation, as well as in related diseases and conditions, such as ischemic reperfusion injury, AIDS, and cancer. Structure Like other cyclophilins, PPID forms a β-barrel structure with a hydrophobic core. This β-barrel is composed of eight anti-parallel β-strands and capped by two α-helices at the top and bottom. In addition, the β-turns and loops in the strands contribute to the flexibility of the barrel. PPID in particular is composed of 370 residues and shares structural homology with PPIF, FKBP4, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PPIF
Peptidyl-prolyl cis-trans isomerase, mitochondrial (PPIF) is an enzyme that in humans is encoded by the ''PPIF'' gene. It has also been referred to as, but should not be confused with, cyclophilin D (CypD), which is encoded by the '' PPID'' gene. As a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family, this protein catalyzes the cis-trans isomerization of proline imidic peptide bonds, which allows it to facilitate folding or repair of proteins. PPIF is a major component of the mitochondrial permeability transition pore (MPTP) and, thus, highly involved in mitochondrial metabolism and apoptosis, as well as in mitochondrial diseases and related conditions, including cardiac diseases, neurodegenerative diseases, and muscular dystrophy. In addition, PPIF participates in inflammation, as well as in ischemic reperfusion injury, AIDS, and cancer. Structure Like other cyclophilins, PPIF forms a β-barrel structure with a hydrophobic core. This β-barrel is composed of ei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminal
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that j ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Oxygen Species
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen. The reduction of molecular oxygen () produces superoxide (), which is the precursor to most other reactive oxygen species: :O2 + e^- -> \ ^\bullet O2- Dismutation of superoxide produces hydrogen peroxide (): :2 H+ + \ ^\bullet O2^- + \ ^\bullet O2^- -> H2O2 + O2 Hydrogen peroxide in turn may be partially reduced, thus forming hydroxide ions and hydroxyl radicals (), or fully reduced to water: :H2O2 + e^- -> HO^- + \ ^\bullet OH :2 H+ + 2 e- + H2O2 -> 2 H2O In a biological context, ROS are byproducts of the normal metabolism of oxygen. ROS have roles in cell signaling and homeostasis. ROS are intrinsic to cellular functioning, and are present at low and stationary levels in normal cells. In plants, ROS are involved in metabolic processes related to photoprotection and toleran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hsp90
Hsp90 (heat shock protein 90) is a chaperone protein that assists other proteins to fold properly, stabilizes proteins against heat stress, and aids in protein degradation. It also stabilizes a number of proteins required for tumor growth, which is why Hsp90 inhibitors are investigated as anti-cancer drugs. Heat shock proteins, as a class, are among the most highly expressed cellular proteins across all species. As their name implies, heat shock proteins protect cells when stressed by elevated temperatures. They account for 1–2% of total protein in unstressed cells. However, when cells are heated, the fraction of heat shock proteins increases to 4–6% of cellular proteins. Heat shock protein 90 (Hsp90) is one of the most common of the heat-related proteins. The "90" comes from the fact that it has a mass of roughly 90 kilodaltons. A 90 kDa protein is considered fairly large for a non-fibrous protein. Hsp90 is found in bacteria and all branches of eukarya ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclosporin A
Ciclosporin, also spelled cyclosporine and cyclosporin, is a calcineurin inhibitor, used as an immunosuppressant medication. It is a natural product. It is taken orally or intravenously for rheumatoid arthritis, psoriasis, Crohn's disease, nephrotic syndrome, and in organ transplants to prevent rejection. It is also used as eye drops for keratoconjunctivitis sicca (dry eyes). Common side effects include high blood pressure, headache, kidney problems, increased hair growth, and vomiting. Other severe side effects include an increased risk of infection, liver problems, and an increased risk of lymphoma. Blood levels of the medication should be checked to decrease the risk of side effects. Use during pregnancy may result in preterm birth; however, ciclosporin does not appear to cause birth defects. Ciclosporin is believed to work by decreasing the function of lymphocytes. It does this by forming a complex with cyclophilin to block the phosphatase activity of calcineurin, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parvulin
] Parvulin, a 92-amino acid protein discovered in E. coli in 1994,Rahfeld JU, Schierhorn A, Mann KH. (1994). A novel peptidyl-prolyl cis/trans isomerase from Escherichia coli. ''FEBS Lett'' 343:65. is the smallest known protein with prolyl isomerase activity, which catalyzes the ''cis''-''trans'' isomerization of proline peptide bonds. Although parvulin has no homology with larger prolyl isomerases such as cyclophilin and FKBP, it does share structural features with subdomains of other proteins involved in preparing secreted proteins for export from the cell.Balbach J, Schmid FX. (2000). Proline isomerizarion and its catalysis in protein folding. ''In Mechanisms of Protein Folding'' 2nd ed. Ed. RH Pain. ''Frontiers in Molecular Biology'' series. Oxford University Press: Oxford, UK. Although parvulin is as active as the larger prolyl isomerases against a short proline-containing test peptide, it has lower relative activity against biological substrates, possibly because the larger ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FKBP
FKBP, or FK506 binding protein, is a family of proteins that have prolyl isomerase activity and are related to the cyclophilins in function, though not in amino acid sequence. FKBPs have been identified in many eukaryotes, ranging from yeast to humans, and function as protein folding chaperones for proteins containing proline residues. Along with cyclophilin, FKBPs belong to the immunophilin family. FKBP12 is notable in humans for binding the immunosuppressant molecule tacrolimus (originally designated FK506), which is used in treating patients after organ transplant and patients with autoimmune disorders. Tacrolimus has been found to reduce episodes of organ rejection over a related treatment, the drug ciclosporin, which binds cyclophilin. Both the FKBP-tacrolimus complex and the cyclosporin-cyclophilin complex inhibit a phosphatase called calcineurin, thus blocking signal transduction in the T-lymphocyte transduction pathway. This therapeutic role is not related to prolyl isome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclophilin
Cyclophilins (CYPs) are a family of proteins named after their ability to bind to ciclosporin (cyclosporin A), an immunosuppressant which is usually used to suppress rejection after internal organ transplants. They are found in all domains of life. These proteins have peptidyl prolyl isomerase activity, which catalyzes the isomerization of peptide bonds from ''trans'' form to ''cis'' form at proline residues and facilitates protein folding. Cyclophilin A is a cytosolic and highly abundant protein. The protein belongs to a family of isozymes, including cyclophilins B and C, and natural killer cell cyclophilin-related protein. Major isoforms have been found within single cells, including inside the Endoplasmic reticulum, and some are even secreted. Mammalian cyclophilins Human genes encoding proteins containing the cyclophilin domain include: * PPIA, PPIB, PPIC, PPID, PPIE, PPIF, PPIG, PPIH * PPIL1, PPIL2, PPIL3, PPIL4, PPIAL4, PPIL6 * PPWD1 Cyclophilin A Cyclophilin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Folding
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproducible process, a polypeptide folds into its characteristic three-dimensional structure from a random coil. Each protein exists first as an unfolded polypeptide or random coil after being translated from a sequence of mRNA to a linear chain of amino acids. At this stage the polypeptide lacks any stable (long-lasting) three-dimensional structure (the left hand side of the first figure). As the polypeptide chain is being synthesized by a ribosome, the linear chain begins to fold into its three-dimensional structure. Folding of many proteins begins even during translation of the polypeptide chain. Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oligopeptide
An oligopeptide, often just called peptide ('' oligo-'', "a few"), consists of two to twenty amino acids and can include dipeptides, tripeptides, tetrapeptides, and pentapeptides. Some of the major classes of naturally occurring oligopeptides include aeruginosins, cyanopeptolins, microcystins, microviridins, microginins, anabaenopeptins, and cyclamides. Microcystins are best studied, because of their potential toxicity impact in drinking water. A review of some oligopeptides found that the largest class are the cyanopeptolins (40.1%), followed by microcystins (13.4%). Production Oligopeptide classes are produced by nonribosomal peptides synthases (NRPS), except cyclamides and microviridins are synthesized through ribosomic pathways. Examples Examples of oligopeptides include: * Amanitins - Cyclic peptides taken from carpophores of several different mushroom species. They are potent inhibitors of RNA polymerases in most eukaryotic species, the prevent the production of mRNA a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
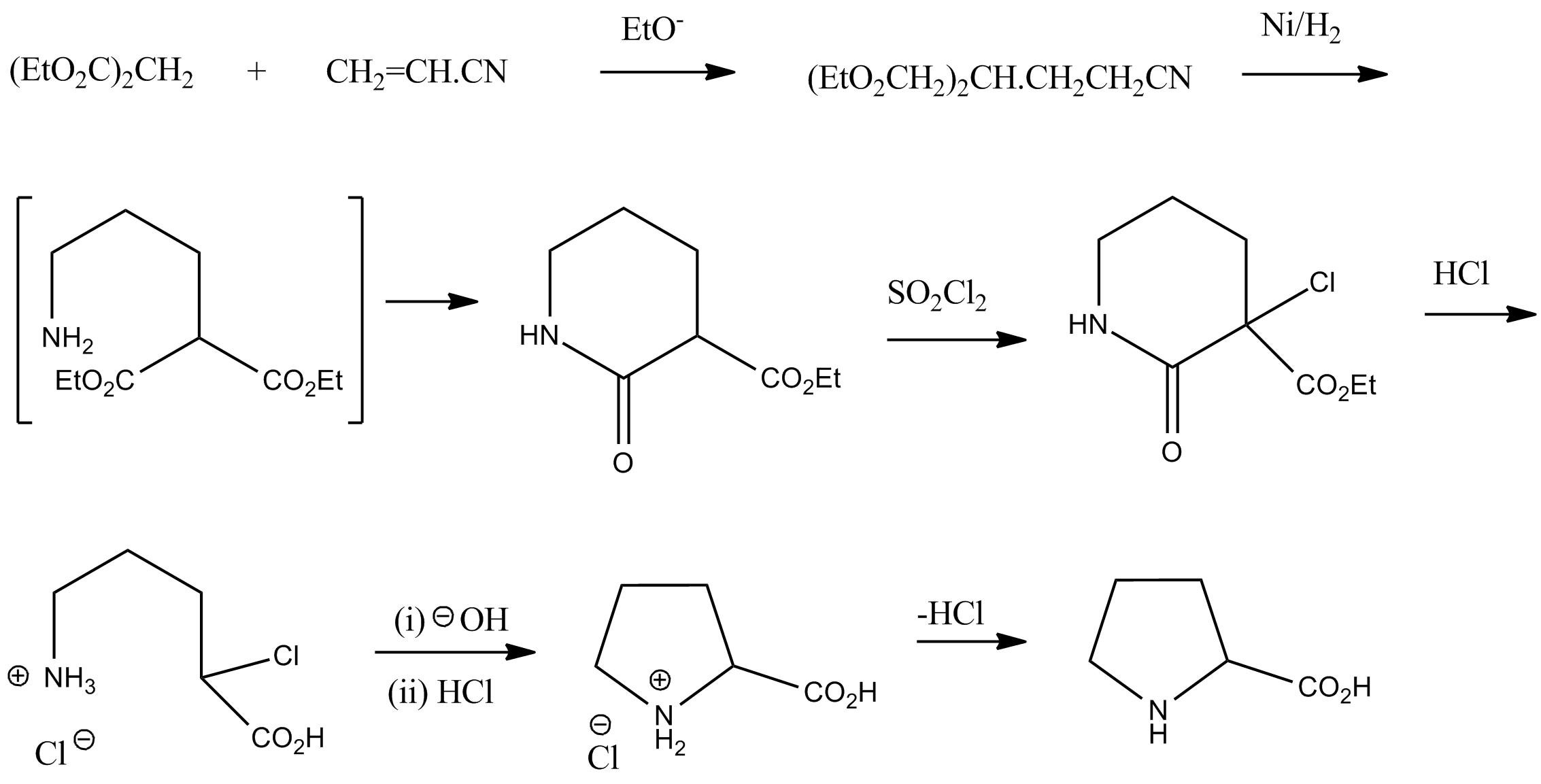
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG). Proline is the only proteinogenic secondary amino acid which is a secondary amine, as the nitrogen atom is attached both to the α-carbon and to a chain of three carbons that together form a five-membered ring. History and etymology Proline was first isolated in 1900 by Richard Willstätter who obtained the amino ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG). Proline is the only proteinogenic secondary amino acid which is a secondary amine, as the nitrogen atom is attached both to the α-carbon and to a chain of three carbons that together form a five-membered ring. History and etymology Proline was first isolated in 1900 by Richard Willstätter who obtained the amino ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |