|
Oligopeptide
An oligopeptide, often just called peptide ('' oligo-'', "a few"), consists of two to twenty amino acids and can include dipeptides, tripeptides, tetrapeptides, and pentapeptides. Some of the major classes of naturally occurring oligopeptides include aeruginosins, cyanopeptolins, microcystins, microviridins, microginins, anabaenopeptins, and cyclamides. Microcystins are best studied, because of their potential toxicity impact in drinking water. A review of some oligopeptides found that the largest class are the cyanopeptolins (40.1%), followed by microcystins (13.4%). Production Oligopeptide classes are produced by nonribosomal peptides synthases (NRPS), except cyclamides and microviridins are synthesized through ribosomic pathways. Examples Examples of oligopeptides include: * Amanitins - Cyclic peptides taken from carpophores of several different mushroom species. They are potent inhibitors of RNA polymerases in most eukaryotic species, the prevent the production of mRNA a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanopeptolin
Cyanopeptolins (CPs) are a class of oligopeptides produced by Microcystis and Planktothrix algae strains, and can be neurotoxic. The production of cyanopeptolins occurs through nonribosomal peptides synthases (NRPS). Chemistry CPs are, in general, a six-residue peptide formed into a ring by a beta-lactone bridge, making them chemically depsipeptides (peptidolactones). The first position is usually threonine, which links to one or two residues via an ester bound on the beta-hydroxyl group; the third position is conserved to be 3-amino-6-hydroxy-2-piperidone (Ahp) or a derivative. All other positions are highly variable. There is not a single, unified nomenclature, for CPs. Names such as CP1020 and CP1138 refer to the molar mass. Others, such as aeruginopeptins, micropeptins, microcystilide, nostopeptins, and oscillapeptins, refer to the organism the substance is originally found in. Factors affecting production Increased water temperatures, because of climate change and eutrophica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclamide
Cyclamides are a class of oligopeptides produced by cyanobacteria algae strains such as ''Microcystis aeruginosa''. Some of them can be toxic. Cyclamides are cyclopeptides with either six or eight amino acids, some of which are modified from the their natural proteinogenic form. They are typically characterized by thiazole and oxazole rings which are thought to be cysteine and threonine derivatives, respectively. Cyclamides are biosynthesized through ribosomic pathways. See also *Cyanopeptolin *Microcystin Microcystins—or cyanoginosins—are a class of toxins produced by certain freshwater cyanobacteria, commonly known as blue-green algae. Over 250 different microcystins have been discovered so far, of which microcystin-LR is the most common. Che ... References External links * {{Cyanotoxins Peptides Cyanotoxins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
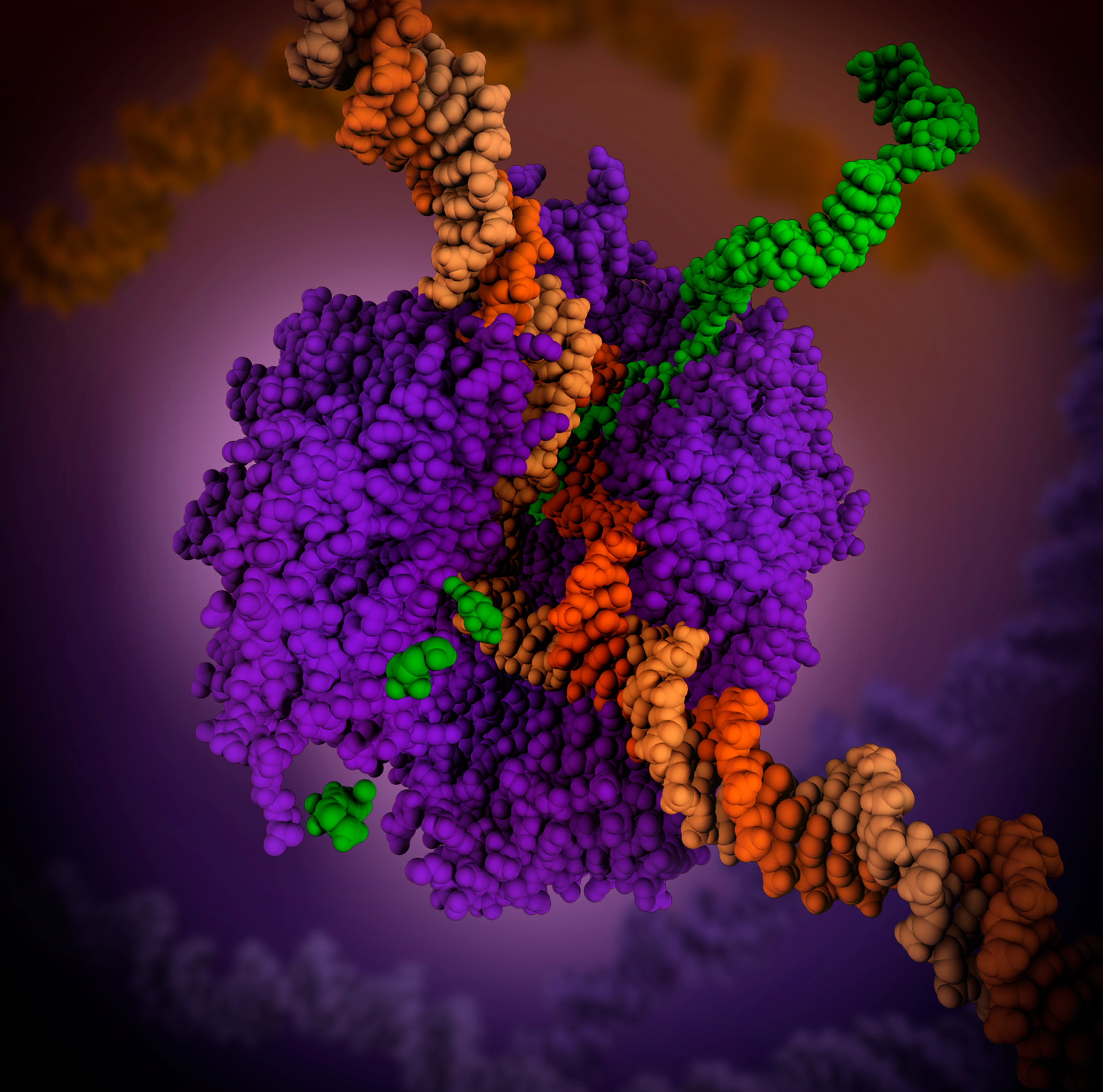
Microcystin
Microcystins—or cyanoginosins—are a class of toxins produced by certain freshwater cyanobacteria, commonly known as blue-green algae. Over 250 different microcystins have been discovered so far, of which microcystin-LR is the most common. Chemically they are cyclic heptapeptides produced through nonribosomal peptide synthases. Cyanobacteria can produce microcystins in large quantities during algal blooms which then pose a major threat to drinking and irrigation water supplies, and the environment at large. Characteristics Microcystins—or cyanoginosins—are a class of toxins produced by certain freshwater cyanobacteria; primarily ''Microcystis aeruginosa'' but also other ''Microcystis'', as well as members of the ''Planktothrix'', ''Anabaena'', ''Oscillatoria'' and ''Nostoc'' genera. Over 250 different microcystins have been discovered so far, of which microcystin-LR is the most common. Chemically they are cyclic heptapeptides produced through nonribosomal peptide synthase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tripeptide Val-Gly-Ala Formula V1
A tripeptide is a peptide derived from three amino acids joined by two or sometimes three peptide bonds. As for proteins, the function of peptides is determined by the constituent amino acids and their sequence. The simplest tripeptide is glycine. In terms of scientific investigations, the dominant tripeptide is glutathione (γ-L-Glutamyl-L-cysteinylglycine), which serves many roles in many forms of life. Examples * Eisenin (pGlu-Gln-Ala-OH) is a peptide with immunological activity that is isolated from the Japanese marine alga, ''Eisenia bicyclis'', which more commonly is known as Arame * GHK-Cu (glycyl-L-histidyl-L-lysine) is a human copper binding peptide with wound healing and skin remodeling activity, which is used in anti-aging cosmetics and more commonly referred to as copper peptide *Lactotripeptides (Ile-Pro-Pro and Val-Pro-Pro) found in milk products, act as ACE inhibitors *Leupeptin (''N''-acetyl-L-leucyl-L-leucyl-L-argininal) is a protease inhibitor that also acts a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antipain
Antipain is an oligopeptide that is isolated from actinomycetes and used in biochemical research as a protease inhibitor of trypsin and papain. It was discovered in 1972 and was the first natural peptide found that contained an ureylene group. Antipain can aid in prevention of coagulation in blood. It is an inhibitor of serine and cysteine proteases. It has been crystallised in complexes with carboxypeptidase, which is obtained from wheat, and ''Leishmania major'' oligopeptidase B. In both cases, the backbone carbonyl of the terminal arginine of antipain forms a covalent bond to the active site serine in the protease. In oncology, pain control is an ongoing problem. An important obstacle to controlling cancer pain is related to the patient. A recent research article indicated that antipain Y is a new type of anti-pain analog, which can inhibit the release of neurotransmitter in rat dorsal root ganglion neurons. An experiment focused on potential improvement for pain management ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism (breakdown of old proteins), and cell signaling. In the absence of functional accelerants, proteolysis would be very slow, taking hundreds of years. Proteases can be found in all forms of life and viruses. They have independently evolved multiple times, and different classes of protease can perform the same reaction by completely different catalytic mechanisms. Hierarchy of proteases Based on catalytic residue Proteases can be classified into seven broad groups: * Serine protease ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ceruletide
Ceruletide (INN), also known as cerulein or caerulein, is a ten amino acid oligopeptide that stimulates smooth muscle and increases digestive secretions. Ceruletide is similar in action and composition to cholecystokinin. It stimulates gastric, biliary, and pancreatic secretion; and certain smooth muscle. It is used in paralytic ileus and as diagnostic aid in pancreatic malfunction. It is used to induce pancreatitis in experimental animal models. Ceruletide was discovered and its structure elucidated in 1967 by Australian and Italian scientists from dried skins of the Australian green tree frog (''Ranoidea caerulea'', formerly ''Hyla caerulea''). Its amino acid sequence is Pglu-Gln-Asp-Tyr O3HThr-Gly-Trp-Met-Asp-Phe-NH2. Induction of pancreatitis Ceruletide upregulates pancreatic acinar cell intercellular adhesion molecule-1 (ICAM-1) proteins through intracellular upregulation of NF-κB. Surface ICAM-1 in turn promotes neutrophil adhesion onto acinar cells enhancing pancrea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Australian Green Tree Frog
The Australian green tree frog (''Ranoidea caerulea''), also known as simply green tree frog in Australia, White's tree frog, or dumpy tree frog, is a species of tree frog native to Australia and New Guinea, with introduced populations in the United States and New Zealand, though the latter is believed to have died out. It is morphologically similar to some other members of its genus, particularly the magnificent tree frog (''R. splendida'') and the white-lipped tree frog (''R. infrafrenata''). Larger than most Australian frogs, the Australian green tree frog reaches 10 cm (4 in) or more in length. Its average lifespan in captivity, about 16 years, is long compared with most frogs. Docile and well suited to living near human dwellings, Australian green tree frogs are often found on window sills or inside houses, eating insects drawn by the light. The green tree frog screams when it is in danger to scare off its foe, and squeaks when it is touched. Due to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholecystokinin
Cholecystokinin (CCK or CCK-PZ; from Greek ''chole'', "bile"; ''cysto'', "sac"; ''kinin'', "move"; hence, ''move the bile-sac (gallbladder)'') is a peptide hormone of the gastrointestinal system responsible for stimulating the digestion of fat and protein. Cholecystokinin, formerly called pancreozymin, is synthesized and secreted by enteroendocrine cells in the duodenum, the first segment of the small intestine. Its presence causes the release of digestive enzymes and bile from the pancreas and gallbladder, respectively, and also acts as a hunger suppressant. History Evidence that the small intestine controls the release of bile was uncovered as early as 1856, when French physiologist Claude Bernard showed that when dilute acetic acid was applied to the orifice of the bile duct, the duct released bile into the duodenum. In 1903 the French physiologist showed that this reflex was not mediated by the nervous system. In 1904 the French physiologist Charles Fleig showed that the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RNA Polymerase
In molecular biology, RNA polymerase (abbreviated RNAP or RNApol), or more specifically DNA-directed/dependent RNA polymerase (DdRP), is an enzyme that synthesizes RNA from a DNA template. Using the enzyme helicase, RNAP locally opens the double-stranded DNA so that one strand of the exposed nucleotides can be used as a template for the synthesis of RNA, a process called transcription. A transcription factor and its associated transcription mediator complex must be attached to a DNA binding site called a promoter region before RNAP can initiate the DNA unwinding at that position. RNAP not only initiates RNA transcription, it also guides the nucleotides into position, facilitates attachment and elongation, has intrinsic proofreading and replacement capabilities, and termination recognition capability. In eukaryotes, RNAP can build chains as long as 2.4 million nucleotides. RNAP produces RNA that, functionally, is either for protein coding, i.e. messenger RNA (mRNA); or n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
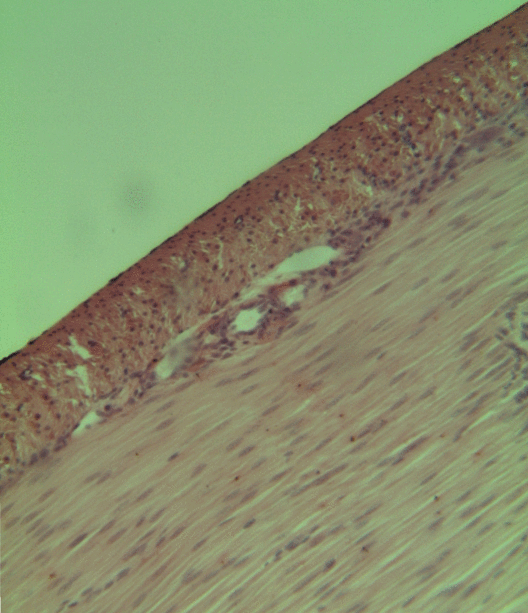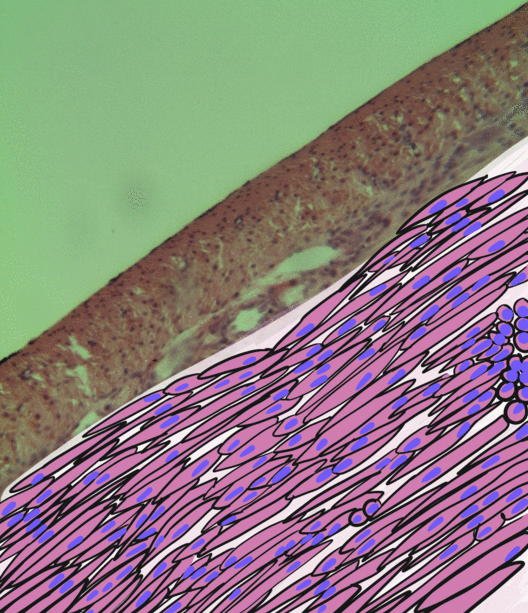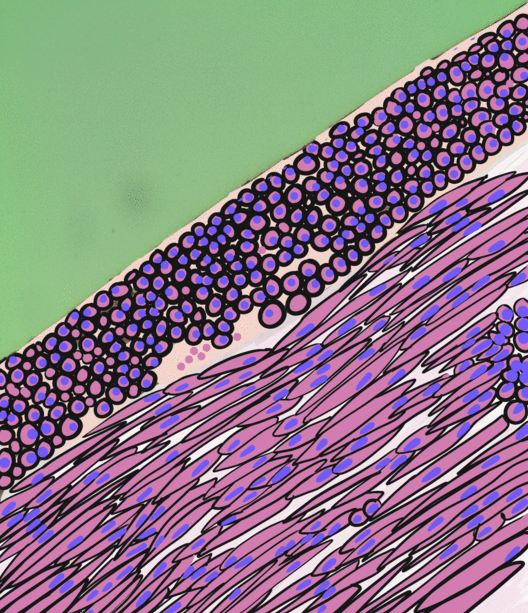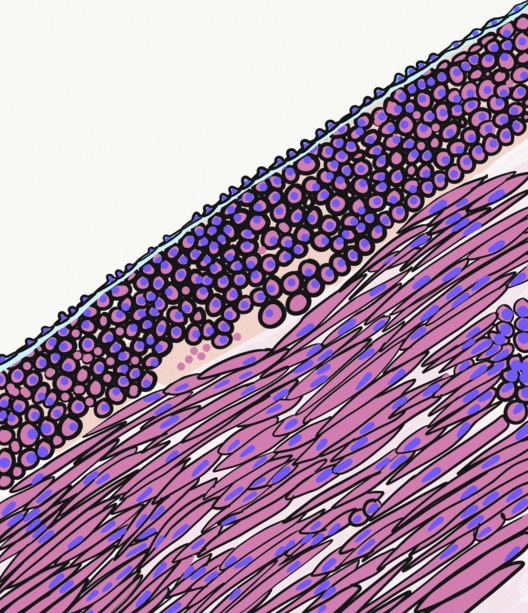
Smooth Muscle
Smooth muscle is an involuntary non-striated muscle, so-called because it has no sarcomeres and therefore no striations (''bands'' or ''stripes''). It is divided into two subgroups, single-unit and multiunit smooth muscle. Within single-unit muscle, the whole bundle or sheet of smooth muscle cells contracts as a syncytium. Smooth muscle is found in the walls of hollow organs, including the stomach, intestines, bladder and uterus; in the walls of passageways, such as blood, and lymph vessels, and in the tracts of the respiratory, urinary, and reproductive systems. In the eyes, the ciliary muscles, a type of smooth muscle, dilate and contract the iris and alter the shape of the lens. In the skin, smooth muscle cells such as those of the arrector pili cause hair to stand erect in response to cold temperature or fear. Structure Gross anatomy Smooth muscle is grouped into two types: single-unit smooth muscle, also known as visceral smooth muscle, and multiunit smooth muscle. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pancreatitis
Pancreatitis is a condition characterized by inflammation of the pancreas. The pancreas is a large organ behind the stomach that produces digestive enzymes and a number of hormones. There are two main types: acute pancreatitis, and chronic pancreatitis. Signs and symptoms of pancreatitis include pain in the upper abdomen, nausea and vomiting. The pain often goes into the back and is usually severe. In acute pancreatitis, a fever may occur, and symptoms typically resolve in a few days. In chronic pancreatitis weight loss, fatty stool, and diarrhea may occur. Complications may include infection, bleeding, diabetes mellitus, or problems with other organs. The two most common causes of acute pancreatitis are a gallstone blocking the common bile duct after the pancreatic duct has joined; and heavy alcohol use. Other causes include direct trauma, certain medications, infections such as mumps, and tumors. Chronic pancreatitis may develop as a result of acute pancreatitis. It is mos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |