|
Cadmium Phosphide
Cadmium phosphide ( Cd3 P2) is an inorganic chemical compound. It is a grey or white bluish solid semiconductor material with a bandgap of 0.5 eV. It has applications as a pesticide, material for laser diodes and for high-power-high-frequency electronics. Synthesis and reactions Cadmium phosphide can be prepared by the reaction of cadmium with phosphorus: :6 Cd + P4 → 2 Cd3P2 Structure Cd3P2 has a room-temperature tetragonal form. The crystalline structure of cadmium phosphide is very similar to that of zinc phosphide (Zn3P2), cadmium arsenide (Cd3As2) and zinc arsenide (Zn3As2). These compounds of the Zn-Cd-P-As quaternary system exhibit full continuous solid-solution. Applications Safety Like other metal phosphides, it is acutely toxic when swallowed due to the formation of phosphine gas when it reacts with gastric acid. It is also carcinogen and dangerous for the skin, eyes and other organs in a large part due to cadmium poisoning. References {{Phospho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base (''a'' by ''a'') and height (''c'', which is different from ''a''). Bravais lattices There are two tetragonal Bravais lattices: the primitive tetragonal and the body-centered tetragonal. The base-centered tetragonal lattice is equivalent to the primitive tetragonal lattice with a smaller unit cell, while the face-centered tetragonal lattice is equivalent to the body-centered tetragonal lattice with a smaller unit cell. Crystal classes The point groups that fall under this crystal system are listed below, followed by their representations in international notation, Schoenflies notation, orbifold notation, Coxeter notation and mineral examples.Hurlbut, Cornelius S.; Klein, Cornelis, 1985, ''Manual of Mineralogy'', 20th ed., p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
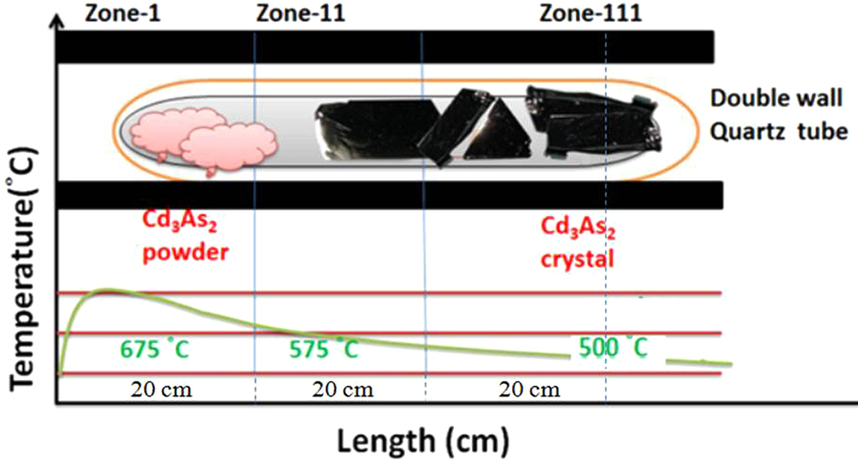
Cadmium Arsenide
Cadmium arsenide ( Cd3 As2) is an inorganic semimetal in the II-V family. It exhibits the Nernst effect. Properties Thermal Cd3As2 dissociates between 220 and 280 °C according to the reaction :2 Cd3As2(s) → 6 Cd(g) + As4(g) An energy barrier was found for the nonstoichiometric vaporization of arsenic due to the irregularity of the partial pressures with temperature. The range of the energy gap is from 0.5 to 0.6 eV. Cd3As2 melts at 716 °C and changes phase at 615 °C/ Phase transition Pure cadmium arsenide undergoes several phase transitions at high temperatures, making phases labeled α (stable), α’, α” (metastable), and β. At 593° the polymorphic transition α → β occurs. :α-Cd3As2 ↔ α’-Cd3As2 occurs at ~500 K. :α’-Cd3As2 ↔ α’’-Cd3As2 occurs at ~742 K and is a regular first order phase transition with marked hysteresis loop. :α”-Cd3As2 ↔ β-Cd3As2 occurs at 868 K. Single crystal x-ray diffraction was used to dete ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Compounds
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it demonstrates oxidation state +2 in most of its compounds, and like mercury, it has a lower melting point than the transition metals in groups 3 through 11. Cadmium and its congeners in group 12 are often not considered transition metals, in that they do not have partly filled ''d'' or ''f'' electron shells in the elemental or common oxidation states. The average concentration of cadmium in Earth's crust is between 0.1 and 0.5 parts per million (ppm). It was discovered in 1817 simultaneously by Stromeyer and Hermann, both in Germany, as an impurity in zinc carbonate. Cadmium occurs as a minor component in most zinc ores and is a byproduct of zinc production. Cadmium was used for a long time as a corrosion-resistant plating on steel, and cadmium compounds are used as red, orange ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphides
In chemistry, a phosphide is a compound containing the ion or its equivalent. Many different phosphides are known, with widely differing structures. Most commonly encountered on the binary phosphides, i.e. those materials consisting only of phosphorus and a less electronegative element. Numerous are polyphosphides, which are solids consisting of anionic chains or clusters of phosphorus. Phosphides are known with the majority of less electronegative elements with the exception of Hg, Pb, Sb, Bi, Te, and Po.Von Schnering, H.G. and Hönle , W. (1994) "Phosphides - Solid-state Chemistry" in ''Encyclopedia of Inorganic Chemistry''. R. Bruce King (ed.). John Wiley & Sons Finally, some phosphides are molecular. Binary phosphides Binary phosphides include phosphorus and one other element. An example of a group 1 phosphide is sodium phosphide (). Other notable examples include aluminium phosphide () and calcium phosphide (), which are used as pesticides, exploiting their tendenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Poisoning
Cadmium is a naturally occurring toxic metal with common exposure in industrial workplaces, plant soils, and from smoking. Due to its low permissible exposure in humans, overexposure may occur even in situations where trace quantities of cadmium are found. Cadmium is used extensively in electroplating, although the nature of the operation does not generally lead to overexposure. Cadmium is also found in some industrial paints and may represent a hazard when sprayed. Operations involving removal of cadmium paints by scraping or blasting may pose a significant hazard. The primary use of cadmium is in the manufacturing of NiCd rechargeable batteries. The primary source for cadmium is as a byproduct of refining zinc metal. Exposures to cadmium are addressed in specific standards for the general industry, shipyard employment, the construction industry, and the agricultural industry. Signs and symptoms Acute Acute exposure to cadmium fumes may cause flu-like symptoms including chi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gastric Acid
Gastric acid, gastric juice, or stomach acid is a digestive fluid formed within the stomach lining. With a pH between 1 and 3, gastric acid plays a key role in digestion of proteins by activating digestive enzymes, which together break down the long chains of amino acids of proteins. Gastric acid is regulated in feedback systems to increase production when needed, such as after a meal. Other cells in the stomach produce bicarbonate, a base, to buffer the fluid, ensuring a regulated pH. These cells also produce mucus – a viscous barrier to prevent gastric acid from damaging the stomach. The pancreas further produces large amounts of bicarbonate and secretes bicarbonate through the pancreatic duct to the duodenum to neutralize gastric acid passing into the digestive tract. The active components of gastric acid are protons and chloride. Often simplistically described as hydrochloric acid, these species are produced by parietal cells in the gastric glands in the stomach. The sec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting fish, due to the presence of substituted phosphine and diphosphane (). With traces of present, is spontaneously flammable in air ( pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure. Phosphines are compounds that include and the organophosphines, which are derived from by substituting one or more hydrogen atoms with organic groups. They have the general formula . Phosphanes are saturated phosphorus hydrides of the form , such as triphosphane. Phosphine, PH3, is the smallest of the phosphines and the smallest of the phosphanes. History Philippe Gengembre (1764–1838), a student of Lavois ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Cadmium Phosphide Arsenide
Zinc cadmium phosphide arsenide ( Zn- Cd- P- As) is a quaternary system of group II (IUPAC group 12) and group V (IUPAC group 15) elements. Many of the inorganic compounds in the system are II-V semiconductor materials. The quaternary system of II3V2 compounds, (Zn1−xCdx)3(P1−yAsy)2, has been shown to allow solid solution continuously over the whole compositional range. This material system and its subsets have applications in electronics, optoelectronics, including photovoltaics, and thermoelectrics. List of all binary compounds This system of elements contains numerous binary compounds and their solid solutions. Stable at atmospheric pressure The binary compounds thermodynamically stable at atmospheric pressure are listed in the following table: Metastable or unstable at atmospheric pressure Compounds metastable or unstable at atmospheric pressure are the following: Quaternary compounds The compounds of the form II3V2 have similar crystalline structures and e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Arsenide
Zinc arsenide (Zn3As2) is a binary compound of zinc with arsenic which forms gray tetragonal crystals. It is an inorganic semiconductor with a band gap of 1.0 eV. Synthesis and reactions Zinc arsenide can be prepared by the reaction of zinc with arsenic :3 Zn + 2 As → Zn3As2 Structure Zn3As2 has a room-temperature tetragonal form that converts to a different tetragonal phase at 190 °C and to a third phase at 651 °C. In the room-temperature form, the zinc atoms are tetrahedrally coordinated and the arsenic atoms are surrounded by six zinc atoms at the vertices of a distorted cube. The crystalline structure of zinc arsenide is very similar to that of cadmium arsenide (Cd3As2), zinc phosphide (Zn3P2) and cadmium phosphide (Cd3P2). These compounds of the Zn-Cd-P-As quaternary system exhibit full continuous solid-solution. Electronic structure Its lowest direct and indirect bandgaps are within 30 meV or each other. References arsenide zinc Zinc i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Phosphide
Zinc phosphide ( Zn3 P2) is an inorganic chemical compound. It is a grey solid, although commercial samples are often dark or even black. It is used as a rodenticide. Zn3P2 is a II-V semiconductor with a direct band gap of 1.5 eV and may have applications in photovoltaic cells. A second compound exists in the zinc-phosphorus system, zinc diphosphide (ZnP2). Synthesis and reactions Zinc phosphide can be prepared by the reaction of zinc with phosphorus; however, for critical applications, additional processing to remove arsenic compounds may be needed. :6 Zn + P4 → 2 Zn3P2 Another method of preparation include reacting tri-n-octylphosphine with dimethylzinc. Zinc phosphide reacts with water to produce phosphine (PH3) and zinc hydroxide (Zn(OH)2): :Zn3P2 + 6 H2O → 2 PH3 + 3 Zn(OH)2 Structure Zn3P2 has a room-temperature tetragonal form that converts to a cubic form at around 845 °C.Evgeniĭ I︠U︡rʹevich Tonkov, 1992, High Pressure Phase Transformati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pearson Symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure, and was originated by W. B. Pearson. The symbol is made up of two letters followed by a number. For example: * Diamond structure, ''cF''8 * Rutile structure, ''tP''6 The two (italicised) letters specify the Bravais lattice. The lower-case letter specifies the crystal family, and the upper-case letter the centering type. The number at the end of the Pearson symbol gives the number of the atoms in the conventional unit cell.Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005 IR-3.4.4, pp. 49–51; IR-11.5, pp. 241–242. |
Tetragonal Crystal System
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base (''a'' by ''a'') and height (''c'', which is different from ''a''). Bravais lattices There are two tetragonal Bravais lattices: the primitive tetragonal and the body-centered tetragonal. The base-centered tetragonal lattice is equivalent to the primitive tetragonal lattice with a smaller unit cell, while the face-centered tetragonal lattice is equivalent to the body-centered tetragonal lattice with a smaller unit cell. Crystal classes The point groups that fall under this crystal system are listed below, followed by their representations in international notation, Schoenflies notation, orbifold notation, Coxeter notation and mineral examples.Hurlbut, Cornelius S.; Klein, Cornelis, 1985, ''Manual of Mineralogy'', 20th ed., pp. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |