|
Carfenazine
Carfenazine (INN) (former developmental code name WY-2445), or carphenazine ( BAN), also known as carphenazine maleate (USAN) (brand name Proketazine; former developmental code name NSC-71755), is an antipsychotic and tranquilizer of the phenothiazine group that was withdrawn from the market. Synthesis The alkylation reaction between 2-Propionyl Phenothiazine 2-33-1(1) and 1-Bromo-3-chloropropane (2) gives 1- 0-(3-chloropropyl)phenothiazin-2-ylropan-1-one 5157-45-2(3). A second alkylation step, this time with 2-(1-Piperazinyl)ethanol 03-76-4(4) completes the synthesis of ''Carfenazine'' (5). NB: Although above procedure is proof-of-concept, bear in mind no protecting group Analogues * Butaperazine uses butanoyl ( Butyryl) and not propanoyl group. *Fluphenazine selfsame but trifluoromethyl on position 2 of the phenothiazine Phenothiazine, abbreviated PTZ, is an organic compound that has the formula S(C6H4)2NH and is related to the thiazine-class of heterocyclic compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butaperazine
Butaperazine (Repoise, Tyrylen) is a typical antipsychotic of the phenothiazine class. It was approved in 1967, and possibly discontinued in the 1980s. Synthesis 2-Butyrylphenothiazine 5244-91-1(1) is the requisite starting material for carrying out the procedure. It is prepared in a manner that is synonymous with the method used in the propiomazine and propiopromazine already discussed. The 1-(γ-chloropropyl)-4-methylpiperazine 04-16-5(2) is prepared in the conventional way from alkylating 1-methylpiperazine and 1-Bromo-3-chloropropane. Sodamide is used to extract the 10-H thereby facilitating the nucleophilic substitution reaction. And completing the instalment of the sidechain. See also * Typical antipsychotic * Phenothiazine Phenothiazine, abbreviated PTZ, is an organic compound that has the formula S(C6H4)2NH and is related to the thiazine-class of heterocyclic compounds. Derivatives of phenothiazine are highly bioactive and have widespread use and rich history ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenothiazines
Phenothiazine, abbreviated PTZ, is an organic compound that has the formula S(C6H4)2NH and is related to the thiazine-class of heterocyclic compounds. Derivatives of phenothiazine are highly bioactive and have widespread use and rich history. The derivatives chlorpromazine and promethazine revolutionized the fields of psychiatry and allergy treatment, respectively. An earlier derivative, methylene blue, was one of the first antimalarial drugs, and derivatives are under investigation as possible anti-infective drugs. Phenothiazine is a prototypical pharmaceutical lead structure in medicinal chemistry. Uses Phenothiazine itself is only of theoretical interest, but its derivatives revolutionized psychiatry, other fields of medicine, and pest management. Other derivatives have been studied for possible use in advanced batteries and fuel cells. Phenothiazine-derived drugs In 1876, methylene blue, a derivative of phenothiazine, was synthesized by Heinrich Caro at BASF. The struc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
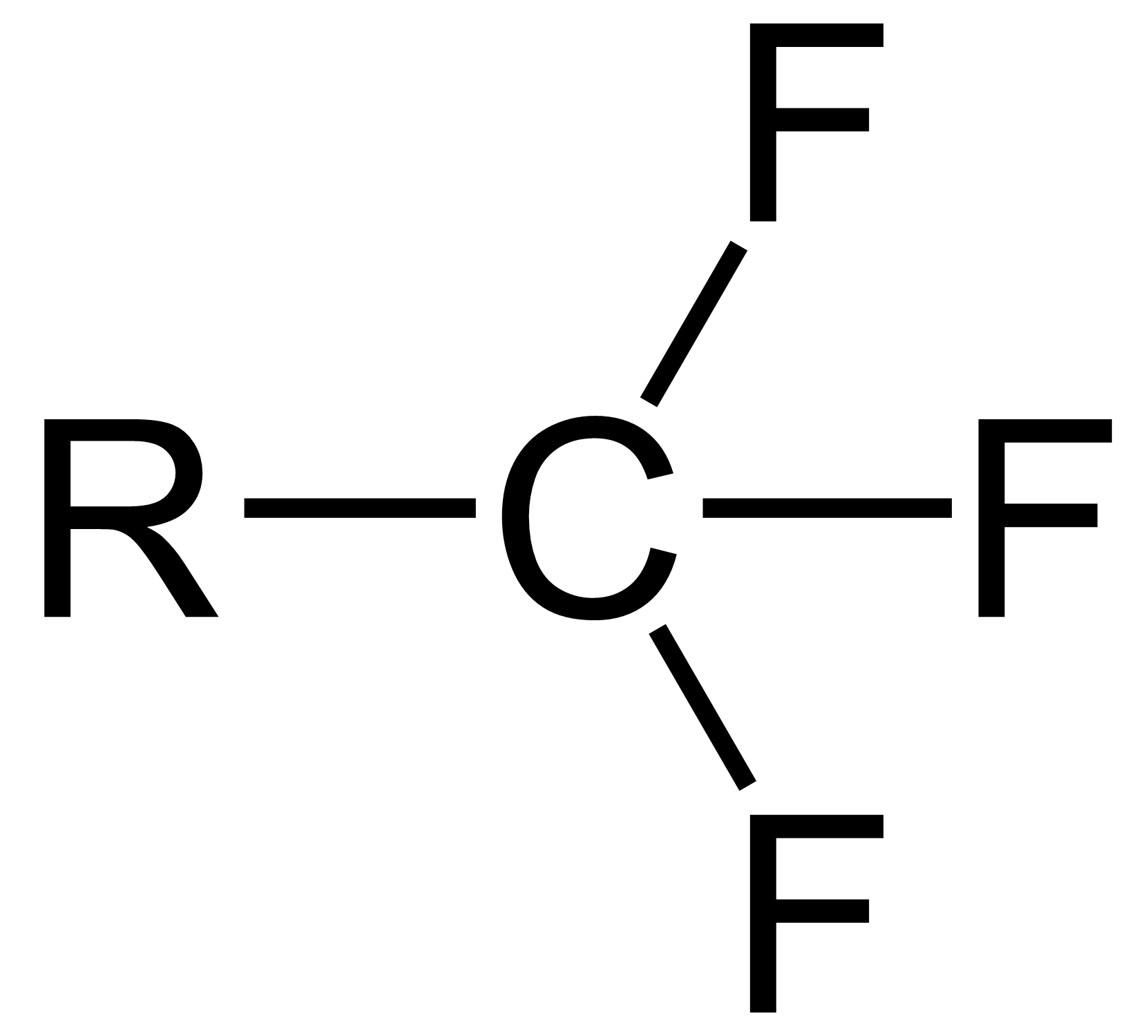
Trifluoromethyl
The trifluoromethyl group is a functional group that has the formula -CF3. The naming of is group is derived from the methyl group (which has the formula -CH3), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane H–, 1,1,1-trifluoroethane –, and hexafluoroacetone –CO–. Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid Trifluoroacetic acid (TFA) is an organofluorine compound with the chemical formula CF3CO2H. It is a structural analogue of acetic acid with all three of the acetyl group's hydrogen atoms replaced by fluorine atoms and is a colorless liquid with a .... Conversely, the trifluoromethyl group ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluphenazine
Fluphenazine, sold under the brand name Prolixin among others, is a high-potency typical antipsychotic medication. It is used in the treatment of chronic psychoses such as schizophrenia, and appears to be about equal in effectiveness to low-potency antipsychotics like chlorpromazine. It is given by mouth, injection into a muscle, or just under the skin. There is also a long acting injectable version that may last for up to four weeks. Fluphenazine decanoate, the depot injection form of fluphenazine, should not be used by people with severe depression. Common side effects include movement problems, sleepiness, depression and increased weight. Serious side effects may include neuroleptic malignant syndrome, low white blood cell levels, and the potentially permanent movement disorder tardive dyskinesia. In older people with psychosis as a result of dementia it may increase the risk of dying. It may also increase prolactin levels which may result in milk production, enlarged b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propanoyl
Propionic acid (, from the Greek words πρῶτος : ''prōtos'', meaning "first", and πίων : ''píōn'', meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula CH3CH2CO2H. It is a liquid with a pungent and unpleasant smell somewhat resembling body odor. The anion CH3CH2CO2− as well as the salts and esters of propionic acid are known as propionates or propanoates. History Propionic acid was first described in 1844 by Johann Gottlieb, who found it among the degradation products of sugar. Over the next few years, other chemists produced propionic acid by different means, none of them realizing they were producing the same substance. In 1847, French chemist Jean-Baptiste Dumas established all the acids to be the same compound, which he called propionic acid, from the Greek words πρῶτος (prōtos), meaning ''first'', and πίων (piōn), meaning ''fat'', because it is the smallest H(CH2)''n''COOH acid that exhibits ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butyryl
Butyric acid (; from grc, βούτῡρον, meaning "butter"), also known under the systematic name butanoic acid, is a straight-chain alkyl carboxylic acid with the chemical formula CH3CH2CH2CO2H. It is an oily, colorless liquid with an unpleasant odor. Isobutyric acid (2-methylpropanoic acid) is an isomer. Salts and esters of butyric acid are known as butyrates or butanoates. The acid does not occur widely in nature, but its esters are widespread. It is a common industrial chemical and an important component in the mammalian gut. History Butyric acid was first observed in impure form in 1814 by the French chemist Michel Eugène Chevreul. By 1818, he had purified it sufficiently to characterize it. However, Chevreul did not publish his early research on butyric acid; instead, he deposited his findings in manuscript form with the secretary of the Academy of Sciences in Paris, France. Henri Braconnot, a French chemist, was also researching the composition of butter and was publi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protecting Group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis. In many preparations of delicate organic compounds, some specific parts of their molecules cannot survive the required reagents or chemical environments. Then, these parts, or groups, must be protected. For example, lithium aluminium hydride is a highly reactive but useful reagent capable of reducing esters to alcohols. It will always react with carbonyl groups, and this cannot be discouraged by any means. When a reduction of an ester is required in the presence of a carbonyl, the attack of the hydride on the carbonyl has to be prevented. For example, the carbonyl is converted into an acetal, which does not react with hydrides. The acetal is then called a protecting group for the carbonyl. After the step involving the hydride is complete, the acet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
British Approved Name
A British Approved Name (BAN) is the official, non-proprietary, or generic name given to a pharmaceutical substance, as defined in the British Pharmacopoeia (BP). The BAN is also the official name used in some countries around the world, because starting in 1953, proposed new names were evaluated by a panel of experts from WHO in conjunction with the BP commission to ensure naming consistency worldwide (an effort leading to the International Nonproprietary Name system). There is also a British Approved Name (Modified) (BANM). Combination preparations BANs are unique in that names are assigned for combination preparations as well as single-drug preparations. For example, the BAN Co-amoxiclav is assigned to preparations containing amoxicillin and clavulanic acid. Most other pharmacopoeias simply refer to combination products by both ingredients in the preparation, in this example "amoxicillin with clavulanic acid". The prefix of "co-" is used for many combination drugs, including ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nota Bene
(, or ; plural form ) is a Latin phrase meaning "note well". It is often abbreviated as NB, n.b., or with the ligature and first appeared in English writing . In Modern English, it is used, particularly in legal papers, to draw the attention of the reader to a certain (side) aspect or detail of the subject being addressed. While ''NB'' is also often used in academic writing, ''note'' is a common substitute. The markings used to draw readers' attention in medieval manuscripts are also called marks. The common medieval markings do not, however, include the abbreviation ''NB''. The usual medieval equivalents are anagrams from the four letters in the word , the abbreviation DM from ("worth remembering"), or a symbol of a little hand (☞), called a manicule or index, with the index finger pointing towards the beginning of the significant passage.Raymond Clemens and Timothy Graham, Introduction to Manuscript Studies (Ithaca: Cornell University Press, 2007), p. 44. Se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-Bromo-3-chloropropane
1-Bromo-3-chloropropane is an organohalogen compound with the formula Br(CH2)3Cl. It is a colorless liquid, produced by free-radical addition of hydrogen bromide to allyl chloride. It is used as an alkylating agent to install the –(CH2)3Cl and –(CH2)3– groups. For example, it is a precursor to 4-chlorobutyronitrile 4-Chlorobutyronitrile is the organic compound with the formula ClCH2CH2CH2CN. With both chloro and cyano functional groups, it is a bifunctional molecule. This colorless liquid is prepared by the reaction of sodium cyanide with 1-bromo-3-chloropr .... References {{DEFAULTSORT:Bromo-3-chloropropane, 1- Organochlorides Organobromides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |