Trifluoromethyl on:
[Wikipedia]
[Google]
[Amazon]
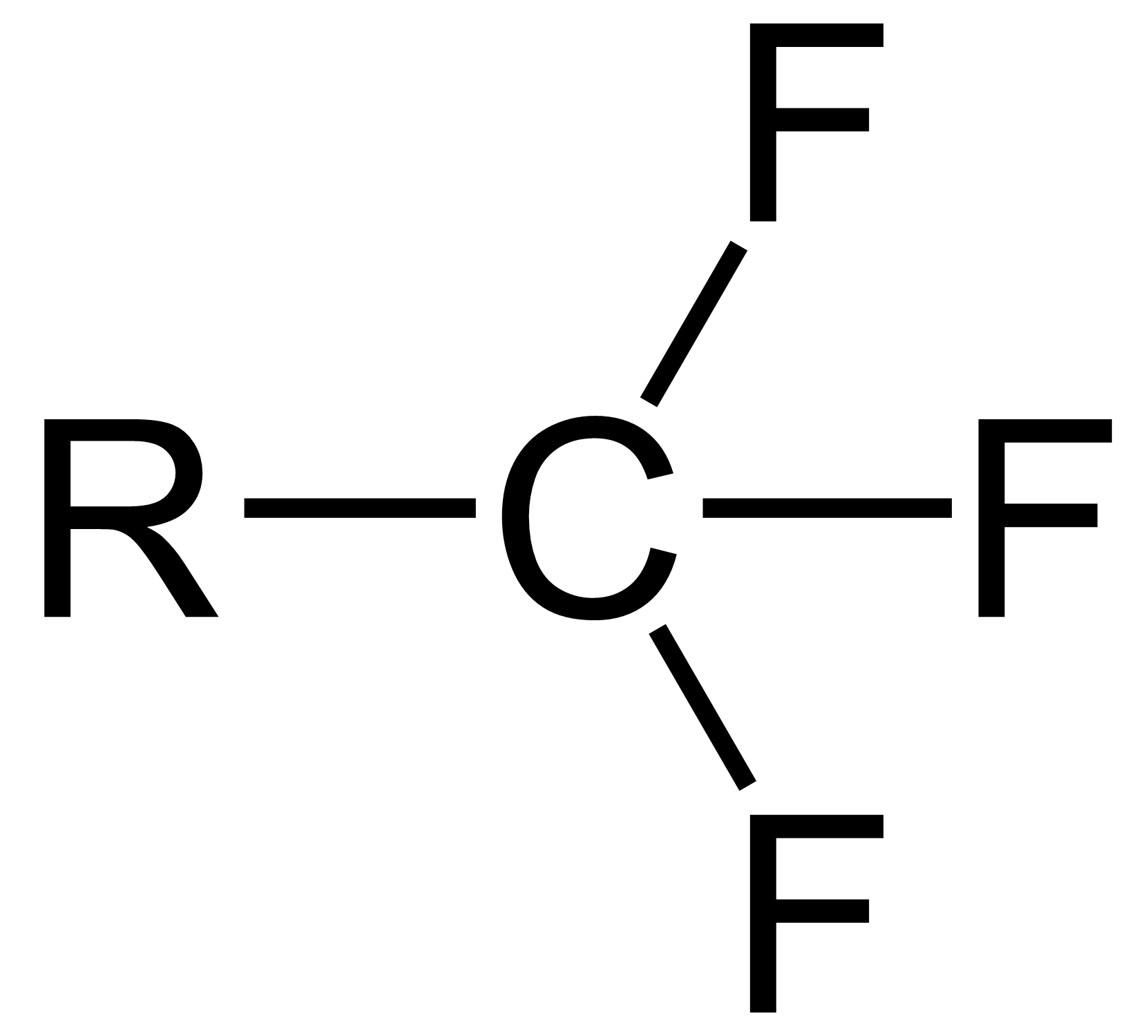 The trifluoromethyl group is a
The trifluoromethyl group is a
 The trifluoromethyl group is a
The trifluoromethyl group is a functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the r ...
that has the formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwe ...
-CF3. The naming of is group is derived from the methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in ...
group (which has the formula -CH3), by replacing each hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
atom by a fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactiv ...
atom. Some common examples are trifluoromethane H–, 1,1,1-trifluoroethane –, and hexafluoroacetone
Hexafluoroacetone (HFA) is a chemical compound with the formula (CF3)2CO. It is structurally similar to acetone; however, its reactivity is markedly different. It a colourless, hygroscopic, nonflammable, highly reactive gas characterized by a mus ...
–CO–. Compounds with this group are a subclass of the organofluorine
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, r ...
s.
Properties
The trifluoromethyl group has a significantelectronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid
Triflic acid, the short name for trifluoromethanesulfonic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest known acids. Triflic acid is mainly used in research as a catalyst for es ...
and trifluoroacetic acid
Trifluoroacetic acid (TFA) is an organofluorine compound with the chemical formula CF3CO2H. It is a structural analogue of acetic acid with all three of the acetyl group's hydrogen atoms replaced by fluorine atoms and is a colorless liquid with ...
. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol.
Uses
The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized naturalfluorocarbon
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often has distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Fluorocarbons and their derivatives are commerci ...
based compounds. The medicinal use of the trifloromethyl group dates from 1928, although research became more intense in the mid-1940s. The trifluoromethyl group is often used as a bioisostere to create derivatives by replacing a chloride or a methyl group. This can be used to adjust the steric and electronic properties of a lead compound, or to protect a reactive methyl group from metabolic oxidation. Some notable drugs containing trifluoromethyl groups include efavirenz
Efavirenz (EFV), sold under the brand names Sustiva among others, is an antiretroviral medication used to treat and prevent HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention after a needle ...
(Sustiva), an HIV reverse transcriptase inhibitor; fluoxetine
Fluoxetine, sold under the brand names Prozac and Sarafem, among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is used for the treatment of major depressive disorder, obsessive–compulsive diso ...
(Prozac), an antidepressant; and celecoxib (Celebrex), a nonsteroidal anti-inflammatory drug
Non-steroidal anti-inflammatory drugs (NSAID) are members of a therapeutic drug class which reduces pain, decreases inflammation, decreases fever, and prevents blood clots. Side effects depend on the specific drug, its dose and duration o ...
. Sulfoxaflor is used as a systemic insecticide.
The trifluoromethyl group can also be added to change the solubility of molecules containing other groups of interest.
Synthesis
Various methods exist to introduce this functionality.Carboxylic acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
can be converted to trifluoromethyl groups by treatment with sulfur tetrafluoride and trihalomethyl compounds, particularly trifluoromethyl ethers and trifluoromethyl aromatics, are converted into trifluoromethyl compounds by treatment with antimony trifluoride/ antimony pentachloride (the Swarts reaction). Another route to trifluoromethyl aromatics is the reaction of aryl iodides with trifluoromethyl copper. Finally, trifluoromethyl carbonyls can be prepared by reaction of aldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
and esters
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
with Ruppert's reagent.{{cite book , editor1=G.A. Olah , editor2=R.D. Chambers , editor3=G.K.S. Prakash , title = Synthetic fluorine chemistry , publisher = John Wiley , year = 1992 , isbn = 0-471-54370-5
See also
* Trifluromethyl cation * Trichloromethyl group * Trifluoromethoxy group * FluoroethylReferences