|
Caprolactone
ε-Caprolactone or simply caprolactone is a lactone (a cyclic ester) possessing a seven-membered ring. Its name is derived from caproic acid. This colorless liquid is miscible with most organic solvents and water. It was once produced on a large scale as a precursor to caprolactam. Production and uses Caprolactone is prepared industrially by Baeyer-Villiger oxidation of cyclohexanone with peracetic acid. Caprolactone is a monomer used in the production of highly specialised polymers. Ring-opening polymerization, for example, gives polycaprolactone. Another polymer is polyglecaprone, used as suture material in surgery. Reactions Although no longer economical, caprolactone was once produced as a precursor to caprolactam. Caprolactone is treated with ammonia at elevated temperatures to give the lactam: :(CH2)5CO2 + NH3 → (CH2)5C(O)NH + H2O Carbonylation of caprolactone gives, after hydrolysis, pimelic acid. The lactone ring is easily opened with nucleophiles including alcohols ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polycaprolactone
Polycaprolactone (PCL) is a biodegradable polyester with a low melting point of around 60 °C and a glass transition temperature of about −60 °C. The most common use of polycaprolactone is in the production of speciality polyurethanes. Polycaprolactones impart good resistance to water, oil, solvent and chlorine to the polyurethane produced. This polymer is often used as an additive for resins to improve their processing characteristics and their end use properties (e.g., impact resistance). Being compatible with a range of other materials, PCL can be mixed with starch to lower its cost and increase biodegradability or it can be added as a polymeric plasticizer to polyvinyl chloride (PVC). Polycaprolactone is also used for splinting, modeling, and as a feedstock for prototyping systems such as fused filament fabrication 3D printers. Synthesis PCL is prepared by ring opening polymerization of ε-caprolactone using a catalyst such as stannous octoate. A wide r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactone
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring. Lactones are formed by intramolecular esterification of the corresponding hydroxycarboxylic acids, which takes place spontaneously when the ring that is formed is five- or six-membered. Lactones with three- or four-membered rings (α-lactones and β-lactones) are very reactive, making their isolation difficult. Special methods are normally required for the laboratory synthesis of small-ring lactones as well as those that contain rings larger than six-membered. Nomenclature Lactones are usually named according to the precursor acid molecule (''aceto'' = 2 carbon atoms, ''propio'' = 3, ''butyro'' = 4, ''valero'' = 5, ''capro'' = 6, etc.), with a ''-lactone'' suffix and a Greek letter prefix that specifies the number of carbon atoms in the heterocycle — that is, the distance between the relevant -OH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
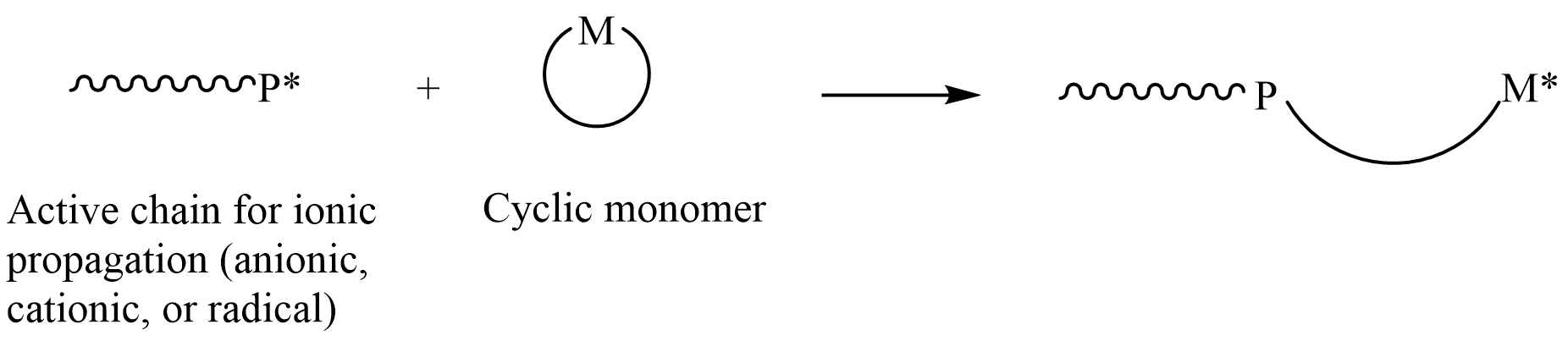
Ring-opening Polymerization
In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization, in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer (see figure). The reactive center can be radical, anionic or cationic. Some cyclic monomers such as norbornene or cyclooctadiene can be polymerized to high molecular weight polymers by using metal catalysts. ROP is a versatile method for the synthesis of biopolymers. Ring-opening of cyclic monomers is often driven by the relief of bond-angle strain. Thus, as is the case for other types of polymerization, the enthalpy change in ring-opening is negative. Monomers Cyclic monomers that are amenable to ROP include epoxides, cyclic trisiloxanes, some lactones, lactides, cyclic carbonates, and amino acid N-carboxyanhydrides. Many strained cycloalkenes, e.g norbornene, are suitable monomers via ring-opening metathesis polymerization. History Ring-opening polymerization has been used since the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pimelic Acid
Pimelic acid is the organic compound with the formula HO2C(CH2)5CO2H. Pimelic acid is one unit longer than a related dicarboxylic acid, adipic acid, a precursor to many polyesters and polyamides. However compared to adipic acid, pimelic acid is relatively small in importance industrially. Derivatives of pimelic acid are involved in the biosynthesis of the amino acid lysine and the vitamin biotin. Synthesis Biosynthesis The biosynthesis of pimelic acid is unknown but is speculated to start with malonyl CoA. Chemical and industrial routes Like other simple dicarboxylic acids, many methods have been developed for producing pimelic acid. Pimelic acid is produced commercially by oxidation of cycloheptanone with dinitrogen tetroxide. Other routes include the relatively unselective oxidation of palmitic acid and the carbonylation of caprolactone. Niche methods Many other methods exist. Pimelic acid has been synthesized from cyclohexanone and from salicylic acid. In the forme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyglecaprone
Monocryl is a synthetic, absorbable suture manufactured in Cornelia, Georgia, USA, and trademarked by Ethicon. It is composed of poliglecaprone 25, which is a copolymer of glycolide and epsilon-caprolactone. It comes both dyed (violet) and undyed (clear) and is an absorbable monofilament suture. It is generally used for soft-tissue approximation and ligation. It is used frequently for subcuticular dermis closures of the face. It has less of a tendency to exit through the skin after it breaks down, such as Vicryl Vicryl (polyglactin 910) is an absorbable, synthetic, usually braided suture, manufactured by Ethicon Inc., a subsidiary of Johnson and Johnson. A monofilament version is also made for use in ophthalmic practice. It is indicated for soft tissue .... It is contraindicated for use in cardiovascular and neurologic tissues and for usage in ophthalmic and microsurgery. The use of poliglecaprone suture may be inappropriate in patients who are older, malnourished, or debilita ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caprolactam
Caprolactam (CPL) is an organic compound with the formula (CH2)5C(O)NH. This colourless solid is a lactam (a cyclic amide) of caproic acid. Global demand for this compound is approximately five million tons per year, and the vast majority is used to make Nylon 6 filament, fiber, and plastics. Synthesis and production Caprolactam was first described in the late 1800s when it was prepared by the cyclization of ε-aminocaproic acid, the product of the hydrolysis of caprolactam. World demand for caprolactam was estimated to reach five million tons per year for 2015. 90% of caprolactam produced is used to make filament and fiber, 10% for plastics, and a small amount is used as a chemical intermediate. Due to its commercial significance, many methods have been developed for the production of caprolactam. It was estimated that 90% of all caprolactam is synthesised from cyclohexanone (1), which is first converted to its oxime (2). Treatment of this oxime with acid induces the Beckmann rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has an odor reminiscent of acetone. Over time, samples of cyclohexanone assume a pale yellow color. Cyclohexanone is slightly soluble in water and miscible with common organic solvents. Billions of kilograms are produced annually, mainly as a precursor to nylon. Production Cyclohexanone is produced by the oxidation of cyclohexane in air, typically using cobalt catalysts: :C6H12 + O2 → (CH2)5CO + H2O This process forms cyclohexanol as a by-product, and this mixture, called "KA Oil" for ketone-alcohol oil, is the main feedstock for the production of adipic acid. The oxidation involves radicals and the hydroperoxide C6H11O2H as an intermediate. In some cases, purified cyclohexanol, obtained by hydration of cyclohexene, is the precursor. Alternatively, cyclohexanone can be produced by the partial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peracetic Acid
Peracetic acid (also known as peroxyacetic acid, or PAA) is an organic compound with the formula CH3CO3H. This peroxy acid is a colorless liquid with a characteristic acrid odor reminiscent of acetic acid. It can be highly corrosive. Peracetic acid is a weaker acid than the parent acetic acid, with a p''K''a of 8.2. Production Peracetic acid is produced industrially by the autoxidation of acetaldehyde: :O2 + CH3CHO → CH3CO3H It forms upon treatment of acetic acid with hydrogen peroxide with a strong acid catalyst: :H2O2 + CH3CO2H CH3CO3H + H2O As an alternative, acetyl chloride and acetic anhydride can be used to generate a solution of the acid with lower water content. Peracetic acid is generated ''in situ'' by some laundry detergents. This is achieved by the action of bleach activators, such as tetraacetylethylenediamine and sodium nonanoyloxybenzenesulfonate, upon hydrogen peroxide formed from sodium percarbonate in water. The peracetic acid is a more effective bleachi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbonylation also refers to oxidation of protein side chains. Organic chemistry Several industrially useful organic chemicals are prepared by carbonylations, which can be highly selective reactions. Carbonylations produce organic carbonyls, i.e., compounds that contain the C=O functional group such as aldehydes, carboxylic acids and esters. Carbonylations are the basis of many types of reactions, including hydroformylation and Reppe reactions. These reactions require metal catalysts, which bind and activate the CO. These processes involve transition metal acyl complexes as intermediates. Much of this theme was developed by Walter Reppe. Hydroformylation Hydroformylation entails the addition of both carbon monoxide and hydrogen to unsaturated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |