|
Caprolactam
Caprolactam (CPL) is an organic compound with the formula (CH2)5C(O)NH. This colourless solid is a lactam (a cyclic amide) of caproic acid. Global demand for this compound is approximately five million tons per year, and the vast majority is used to make Nylon 6 filament, fiber, and plastics. Synthesis and production Caprolactam was first described in the late 1800s when it was prepared by the cyclization of ε-aminocaproic acid, the product of the hydrolysis of caprolactam. World demand for caprolactam was estimated to reach five million tons per year for 2015. 90% of caprolactam produced is used to make filament and fiber, 10% for plastics, and a small amount is used as a chemical intermediate. Due to its commercial significance, many methods have been developed for the production of caprolactam. It was estimated that 90% of all caprolactam is synthesised from cyclohexanone (1), which is first converted to its oxime (2). Treatment of this oxime with acid induces the Beckmann rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caprolactam Polymerization
Caprolactam (CPL) is an organic compound with the formula (CH2)5C(O)NH. This colourless solid is a lactam (a cyclic amide) of caproic acid. Global demand for this compound is approximately five million tons per year, and the vast majority is used to make Nylon 6 filament, fiber, and plastics. Synthesis and production Caprolactam was first described in the late 1800s when it was prepared by the cyclization of ε-aminocaproic acid, the product of the hydrolysis of caprolactam. World demand for caprolactam was estimated to reach five million tons per year for 2015. 90% of caprolactam produced is used to make filament and fiber, 10% for plastics, and a small amount is used as a chemical intermediate. Due to its commercial significance, many methods have been developed for the production of caprolactam. It was estimated that 90% of all caprolactam is synthesised from cyclohexanone (1), which is first converted to its oxime (2). Treatment of this oxime with acid induces the Beckmann rear ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nylon 6
Nylon 6 or polycaprolactam is a polymer, in particular semicrystalline polyamide. Unlike most other nylons, nylon 6 is not a condensation polymer, but instead is formed by ring-opening polymerization; this makes it a special case in the comparison between condensation and addition polymers. Its competition with nylon 6,6 and the example it set have also shaped the economics of the synthetic fibre industry. It is sold under numerous trade names including Perlon (Germany), Dederon (former East Germany), Nylatron, Capron, Ultramid, Akulon, Kapron (former Soviet Union and satellite states), Rugopa (Turkey) and Durethan. History Polycaprolactam was developed by Paul Schlack at IG Farben in late 1930s (first synthesized in 1938) to reproduce the properties of Nylon 66 without violating the patent on its production. (Around the same time, Kohei Hoshino at Toray also succeeded in synthesizing nylon 6.) It was marketed as Perlon, and industrial production with a capacity of 3,500 tons per ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beckmann Rearrangement
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is a rearrangement of an oxime functional group to substituted amides. The rearrangement has also been successfully performed on haloimines and nitrones. Cyclic oximes and haloimines yield lactams. The Beckmann rearrangement is often catalyzed by acid; however, other reagents have been known to promote the rearrangement. These include tosyl chloride, thionyl chloride, phosphorus pentachloride, phosphorus pentoxide, triethylamine, sodium hydroxide, trimethylsilyl iodide among others. The Beckmann fragmentation is another reaction that often competes with the rearrangement, though careful selection of promoting reagent and solvent conditions can favor the formation of one over the other, sometimes giving almost exclusively one product. The rearrangement occurs stereospecifically for ketoximes and N-chloro/N-fluoro imines, with the migrating group being anti-periplanar to the leaving gro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caprolactone
ε-Caprolactone or simply caprolactone is a lactone (a cyclic ester) possessing a seven-membered ring. Its name is derived from caproic acid. This colorless liquid is miscible with most organic solvents and water. It was once produced on a large scale as a precursor to caprolactam. Production and uses Caprolactone is prepared industrially by Baeyer-Villiger oxidation of cyclohexanone with peracetic acid. Caprolactone is a monomer used in the production of highly specialised polymers. Ring-opening polymerization, for example, gives polycaprolactone. Another polymer is polyglecaprone, used as suture material in surgery. Reactions Although no longer economical, caprolactone was once produced as a precursor to caprolactam. Caprolactone is treated with ammonia at elevated temperatures to give the lactam: :(CH2)5CO2 + NH3 → (CH2)5C(O)NH + H2O Carbonylation of caprolactone gives, after hydrolysis, pimelic acid. The lactone ring is easily opened with nucleophiles including alcohols ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexanone Oxime
Cyclohexanone oxime is an organic compound containing the functional group oxime. This colorless solid is an important intermediate in the production of nylon 6, a widely used polymer. Preparation Cyclohexanone oxime can be prepared from the condensation reaction between cyclohexanone and hydroxylamine:J. C. Eck and C. S. Marvel "ε-Benzoylaminocaproic Acid" Org. Synth. 1939, volume 19, pp. 20. :C5H10CO + H2NOH → C5H10C=NOH + H2O Alternatively, another industrial route involves the reaction of cyclohexane with nitrosyl chloride, which is a free radical reaction. This method is advantageous as cyclohexane is much cheaper than cyclohexanone. Reactions The most famous and commercially important reaction of cyclohexanone oxime is Beckmann rearrangement yielding ε-caprolactam: This reaction is catalyzed by sulfuric acid, but industrial scale reactions use solid acids. Typical of oximes, the compound can be reduced by sodium amalgam to produce cyclohexylamine.W. H. Lyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexane is mainly used for the industrial production of adipic acid and caprolactam, which are precursors to nylon. Cyclohexyl () is the alkyl substituent of cyclohexane and is abbreviated Cy. Production Modern On an industrial scale, cyclohexane is produced by hydrogenation of benzene in the presence of a Raney nickel catalyst. Producers of cyclohexane account for approximately 11.4% of global demand for benzene. The reaction is highly exothermic, with ΔH(500 K) = -216.37 kJ/mol. Dehydrogenation commenced noticeably above 300 °C, reflecting the favorable entropy for dehydrogenation. : Early Unlike benzene, cyclohexane is not found in natural resources such as coal. For this reason, early investigators synthesized their cyclohexane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
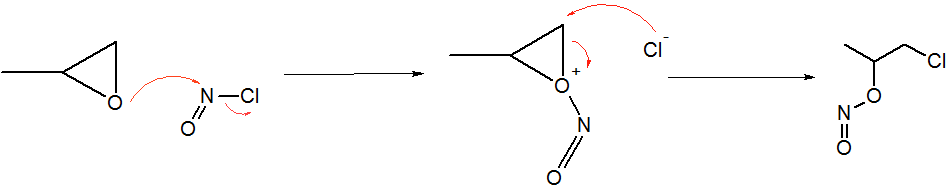
Nitrosyl Chloride
Nitrosyl chloride is the chemical compound with the formula NOCl. It is a yellow gas that is commonly encountered as a component of aqua regia, a mixture of 3 parts concentrated hydrochloric acid and 1 part of concentrated nitric acid. It is a strong electrophile and oxidizing agent. It is sometimes called Tilden's reagent, after William A. Tilden, who was the first to produce it as a pure compound. Structure and synthesis The molecule is bent. A double bond exists between N and O (distance = 1.16 Å) and a single bond between N and Cl (distance = 1.96 Å). The O=N–Cl angle is 113°. Production Nitrosyl chloride can be produced in many ways. * Combining nitrosylsulfuric acid and HCl affords the compound. This method is used industrially. :HCl + NOHSO4 → H2SO4 + NOCl * A more convenient laboratory method involves the (reversible) dehydration of nitrous acid by HCl : HNO2 + HCl → H2O + NOCl * By the direct combination of chlorine and nitric o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactam
A lactam is a cyclic amide, formally derived from an amino alkanoic acid. The term is a portmanteau of the words ''lactone'' + ''amide''. Nomenclature Greek prefixes in alphabetical order indicate ring size: * α-Lactam (3-atom rings) * β-Lactam (4-atom rings) * γ-Lactam (5-atom rings) * δ-Lactam (6-atom rings) * ε-Lactam (7-atom rings) This ring-size nomenclature stems from the fact that a hydrolyzed α-Lactam leads to an α-amino acid and a β-Lactam to a β-amino acid, ''etc''. Synthesis General synthetic methods exist for the organic synthesis of lactams. Beckmann rearrangement Lactams form by the acid-catalyzed rearrangement of oximes in the Beckmann rearrangement. Schmidt reaction Lactams form from cyclic ketones and hydrazoic acid in the Schmidt reaction. Cyclization of amino acids Lactams can be formed from cyclisation of amino acids via the coupling between an amine and a carboxylic acid within the same molecule. Lactamization is most efficient in this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Agency For Research On Cancer
The International Agency for Research on Cancer (IARC; french: Centre International de Recherche sur le Cancer, CIRC) is an intergovernmental agency forming part of the World Health Organization of the United Nations. Its role is to conduct and coordinate research into the causes of cancer. It also collects and publishes surveillance data regarding the occurrence of cancer worldwide. Its IARC monographs programme identifies carcinogenic hazards and evaluates environmental causes of cancer in humans. IARC has its own governing council, and in 1965 the first members were the Federal Republic of Germany, France, Italy, the United Kingdom, and the United States of America. Today, IARC's membership has grown to 27 countries. History In late February 1963, after he experienced his spouse suffering and dying of cancer, journalist and peace activist Yves Poggioli sent a letter to Emmanuel d'Astier de la Vignerie relating his story, and urging support for the creation of an intern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminocaproic Acid
Aminocaproic acid (also known as ε-aminocaproic acid, ε-Ahx, or 6-aminohexanoic acid) is a derivative and analogue of the amino acid lysine, which makes it an effective inhibitor for enzymes that bind that particular residue. Such enzymes include proteolytic enzymes like plasmin, the enzyme responsible for fibrinolysis Fibrinolysis is a process that prevents blood clots from growing and becoming problematic. Primary fibrinolysis is a normal body process, while secondary fibrinolysis is the breakdown of clots due to a medicine, a medical disorder, or some other c .... For this reason it is effective in treatment of certain bleeding disorders, and it is sold under the brand name Amicar. Aminocaproic acid is also an intermediate in the polymerization of Nylon-6, where it is formed by ring-opening hydrolysis of caprolactam. The crystal structure determination showed that the 6-aminohexanoic acid is present as a salt, at least in the solid state. Medical use Aminocaproic acid (Am ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has an odor reminiscent of acetone. Over time, samples of cyclohexanone assume a pale yellow color. Cyclohexanone is slightly soluble in water and miscible with common organic solvents. Billions of kilograms are produced annually, mainly as a precursor to nylon. Production Cyclohexanone is produced by the oxidation of cyclohexane in air, typically using cobalt catalysts: :C6H12 + O2 → (CH2)5CO + H2O This process forms cyclohexanol as a by-product, and this mixture, called "KA Oil" for ketone-alcohol oil, is the main feedstock for the production of adipic acid. The oxidation involves radicals and the hydroperoxide C6H11O2H as an intermediate. In some cases, purified cyclohexanol, obtained by hydration of cyclohexene, is the precursor. Alternatively, cyclohexanone can be produced by the partial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |