|
BORAX
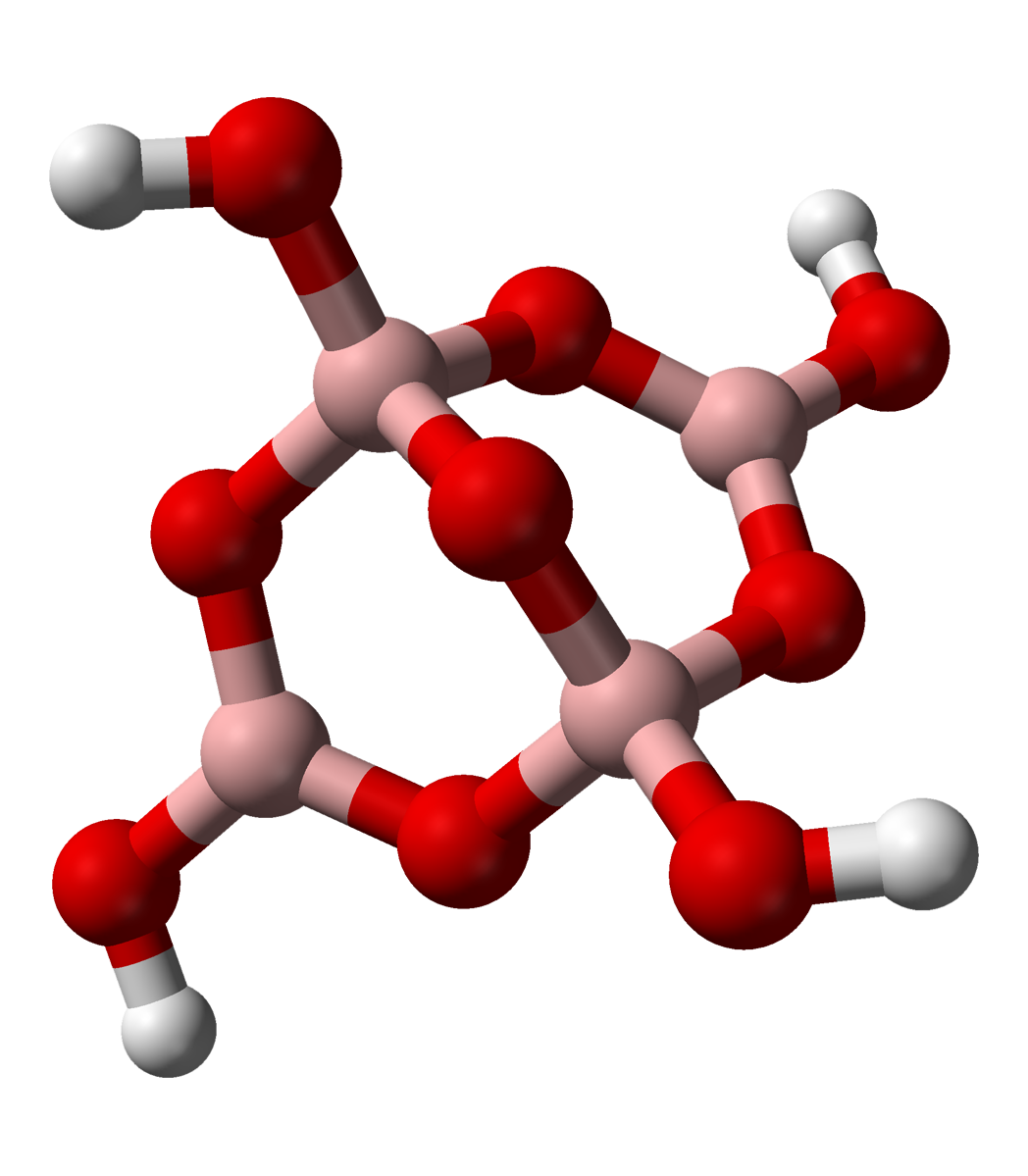
Borax is a salt (ionic compound), a hydrated borate of sodium, with chemical formula often written . It is a colorless crystalline solid, that dissolves in water to make a basic solution. It is commonly available in powder or granular form, and has many industrial and household uses, including as a pesticide, as a metal soldering flux, as a component of glass, enamel, and pottery glazes, for tanning of skins and hides, for artificial aging of wood, as a preservative against wood fungus, and as a pharmaceutic alkalizer. In chemical laboratories, it is used as a buffering agent. The compound is often called sodium tetraborate decahydrate, but that name is not consistent with its structure. The anion is not tetraborate but tetrahydroxy tetraborate , so the more correct formula should be . Informally, the product is often called sodium borate decahydrate or just sodium borate. The terms tincal "tinkle" and tincar "tinker" refer to native borax, historically mined from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral borax, sodium borate, and the ultra-hard crystals of boron carbide and boron nitride. Boron is synthesized entirely by cosmic ray spallation and supernovae and not by stellar nucleosynthesis, so it is a low-abundance element in the Solar System and in the Crust (geology), Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. It is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known deposits are in Turkey, the largest producer of boron minerals. Elemental b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boric Acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen borate or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolves in water, and occurs in nature as the mineral sassolite. It is a weak acid that yields various borate anions and salts, and can react with alcohols to form borate esters. Boric acid is often used as an antiseptic, insecticide, flame retardant, neutron absorber, or precursor to other boron compounds. The term "boric acid" is also used generically for any oxoacid of boron, such as metaboric acid and tetraboric acid . History Orthoboric acid was first prepared by Wilhelm Homberg (1652–1715) from borax, by the action of mineral acids, and was given the name ("sedative salt of Homberg"). However boric acid and borates have been used since the time of the ancient Greeks for cleaning, preserving food, and other activities. Molecular a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flux (metallurgy)
In metallurgy, a flux () is a chemical cleaning agent, flowing agent, or purifying agent. Fluxes may have more than one function at a time. They are used in both extractive metallurgy and metal joining. Some of the earliest known fluxes were sodium carbonate, potash, charcoal, coke, borax, lime, lead sulfide and certain minerals containing phosphorus. Iron ore was also used as a flux in the smelting of copper. These agents served various functions, the simplest being a reducing agent, which prevented oxides from forming on the surface of the molten metal, while others absorbed impurities into the slag, which could be scraped off the molten metal. Fluxes are also used in foundries for removing impurities from molten nonferrous metals such as aluminium, or for adding desirable trace elements such as titanium. As cleaning agents, fluxes facilitate soldering, brazing, and welding by removing oxidation from the metals to be joined. In some applications molten flux also serve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borate
A borate is any of several boron oxyanions, negative ions consisting of boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt with such anions, such as sodium metaborate, and disodium tetraborate . The name also refers to certain functional groups in molecules consisting of boron and oxygen, and esters with such groups, such as triethyl orthoborate . Natural occurrence Borate ions occur, alone or with other anions, in many borate and borosilicate minerals such as borax, boracite, ulexite (boronatrocalcite) and colemanite. Borates also occur in seawater, where they make an important contribution to the absorption of low frequency sound in seawater. Borates also occur in plants, including almost all fruits. Anions The main borate anions are: * tetrahydroxyborate , found in sodium tetrahydroxyborate . * orthoborate , found in trisodium orthoborate * perborate , as in sodium perborate * metaborate or , found in sodium metaborate * diborate , f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraborate
In chemistry, tetraborate or pyroborate is an anion (negative ion) with formula ; or a salt containing that anion, such as sodium tetraborate, . It is one of the boron oxoacids, that is, a borate. The name is also applied to the hydrated ion as present in borax The ion occurs in boric acid solutions at neutral pH, being formed by condensation of orthoborate and tetrahydroxyborate anions: : 2 B(OH)3 + 2 ⇌ + 5 H2O The tetraborate anion (tetramer) includes two tetrahedral and two trigonal boron atoms symmetrically assembled in a fused bicyclic structure. The two tetrahedral boron atoms are linked together by a common oxygen atom, and each also bears a negative net charge brought by the supplementary OH− groups laterally attached to them. This intricate molecular anion also exhibits three rings: two fused distorted hexagonal (boroxole) rings and one distorted octagonal ring. Each ring is made of a succession of alternate boron and oxygen atoms. Boroxole rings are a very comm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Perborate
Sodium perborate is chemical compound whose chemical formula may be written , , or, more properly, ·. Its name is sometimes abbreviated as PBS (not to be confused with phosphate-buffered saline). The compound is commonly encountered in anhydrous form or as a hexahydrate (commonly called "monohydrate" or PBS-1 and "tetrahydrate" or PBS-4, after the early assumption that would be the anhydrous form).Alexander McKillop and William R Sanderson (1995): "Sodium perborate and sodium percarbonate: Cheap, safe and versatile oxidising agents for organic synthesis". ''Tetrahedron'', volume 51, issue 22, pages 6145-6166. They are both white, odorless, water-soluble solids.B.J. Brotherton "Boron: Inorganic Chemistry" in ''Encyclopedia of Inorganic Chemistry'' (1994) Ed. R. Bruce King, John Wiley & Sons This salt is widely used in laundry detergents, as one of the peroxide-based bleaches. Structure Unlike sodium percarbonate and sodium perphosphate, the compound is not simply an adduct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in soap manufacture, and sodium chloride (edible salt) is a de-icing agent and a nutrient for animals including h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Tetraborate
Lithium borate, also known as lithium tetraborate is an inorganic compound with the formula Li2B4O7. A colorless solid, lithium borate is used in making glasses and ceramics. Structure Its structure consists of a polymeric borate backbone. The Li+ centers are bound to four and five oxygen ligands. Boron centers are trigonal and tetrahedral. Lithium borate can be used in the laboratory as LB buffer for gel electrophoresis of DNA and RNA. It is also used in the borax fusion method to vitrify mineral powder specimens for analysis by WDXRF spectroscopy.Ron Jenkins, ''X-Ray Fluorescence Spectrometry, Second Edition'', J. Wiley & Sons Inc., 1999, , p 146-7. See also *LB buffer *Lithium metaborate Lithium metaborate is a chemical compound of lithium, boron, and oxygen with elemental formula . It is often encountered as a hydrate, , where ''n'' is usually 2 or 4. However, these formulas do not describe the actual structure of the solids. ... (LiBO2) References Borates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hide (skin)
A hide or skin is an animal skin treated for human use. The word "hide" is related to the German word "Haut" which means skin. The industry defines hides as "skins" of large animals ''e.g''. cow, buffalo; while skins refer to "skins" of smaller animals: goat, sheep, deer, pig, fish, alligator, snake, etc. Common commercial hides include leather from cattle and other livestock animals, buckskin (leather), buckskin, alligator, alligator skin and snake skin. All are used for shoes, clothes, leather bags, belts, or other fashion Fashion accessory, accessories. Leather is also used in cars, upholstery, interior decorating, horse tack and horse harness, harnesses. Skins are sometimes still gathered from hunting and processed at a domestic or artisanal level but most leather making is now industrialization, industrialized and large-scale. Various tannins are used for this purpose. Hides are also used as processed chews for dogs or other pets. The term "skin" is sometimes expanded to inc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fungus
A fungus ( : fungi or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and molds, as well as the more familiar mushrooms. These organisms are classified as a kingdom, separately from the other eukaryotic kingdoms, which by one traditional classification include Plantae, Animalia, Protozoa, and Chromista. A characteristic that places fungi in a different kingdom from plants, bacteria, and some protists is chitin in their cell walls. Fungi, like animals, are heterotrophs; they acquire their food by absorbing dissolved molecules, typically by secreting digestive enzymes into their environment. Fungi do not photosynthesize. Growth is their means of mobility, except for spores (a few of which are flagellated), which may travel through the air or water. Fungi are the principal decomposers in ecological systems. These and other differences place fungi in a single group of related organisms, named the ''Eumycota'' (''true f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkalizer
Alkalinizing agents are drugs used to manage disorders associated with low pH. For example, they may be used to treat acidosis due to kidney failure. Used for oral or parenteral therapy, sodium bicarbonate is the commonly preferred alkalinizing agent. Others include potassium citrate, calcium carbonate, sodium lactate and calcium acetate Calcium acetate is a chemical compound which is a calcium salt of acetic acid. It has the formula Ca(C2H3O2)2. Its standard name is calcium acetate, while calcium ethanoate is the systematic name. An older name is acetate of lime. The anhydrous .... References {{treatment-stub Drugs acting on the genito-urinary system ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buffer Solution
A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature, there are many living systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also acts as a buffer in the ocean. Principles of buffering Buffer solutions resist pH change because of a chemical equilibrium between the weak acid HA and its conjugate base A−: When some strong acid is added to an equilibrium mixture of the weak acid and its conjugate base, hydrogen ions (H+) are added, and the equilibrium is shifted to the left, in accordance with Le Chatelier's principle. Because of this, the hydrogen io ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |