|
Affinity Tag
Protein tags are peptide sequences genetically grafted onto a recombinant protein. Tags are attached to proteins for various purposes. They can be added to either end of the target protein, so they are either C-terminus or N-terminus specific or are both C-terminus and N-terminus specific. Some tags are also inserted at sites within the protein of interest; they are known as internal tags. Affinity tags are appended to proteins so that they can be purified from their crude biological source using an affinity technique. Affinity tags include chitin binding protein (CBP), maltose binding protein (MBP), Strep-tag and glutathione-S-transferase (GST). The poly(His) tag is a widely used protein tag, which binds to matrices bearing immobilized metal ions. Solubilization tags are used, especially for recombinant proteins expressed in species such as '' E. coli'', to assist in the proper folding in proteins and keep them from aggregating in inclusion bodies. These tags include thior ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Recombinant Protein
Recombinant DNA (rDNA) molecules are DNA molecules formed by laboratory methods of genetic recombination (such as molecular cloning) that bring together genetic material from multiple sources, creating sequences that would not otherwise be found in the genome. Recombinant DNA is the general name for a piece of DNA that has been created by combining at least two fragments from two different sources. Recombinant DNA is possible because DNA molecules from all organisms share the same chemical structure, and differ only in the nucleotide sequence within that identical overall structure. Recombinant DNA molecules are sometimes called chimeric DNA, because they can be made of material from two different species, like the mythical chimera. R-DNA technology uses palindromic sequences and leads to the production of sticky and blunt ends. The DNA sequences used in the construction of recombinant DNA molecules can originate from any species. For example, plant DNA may be joined to bacte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
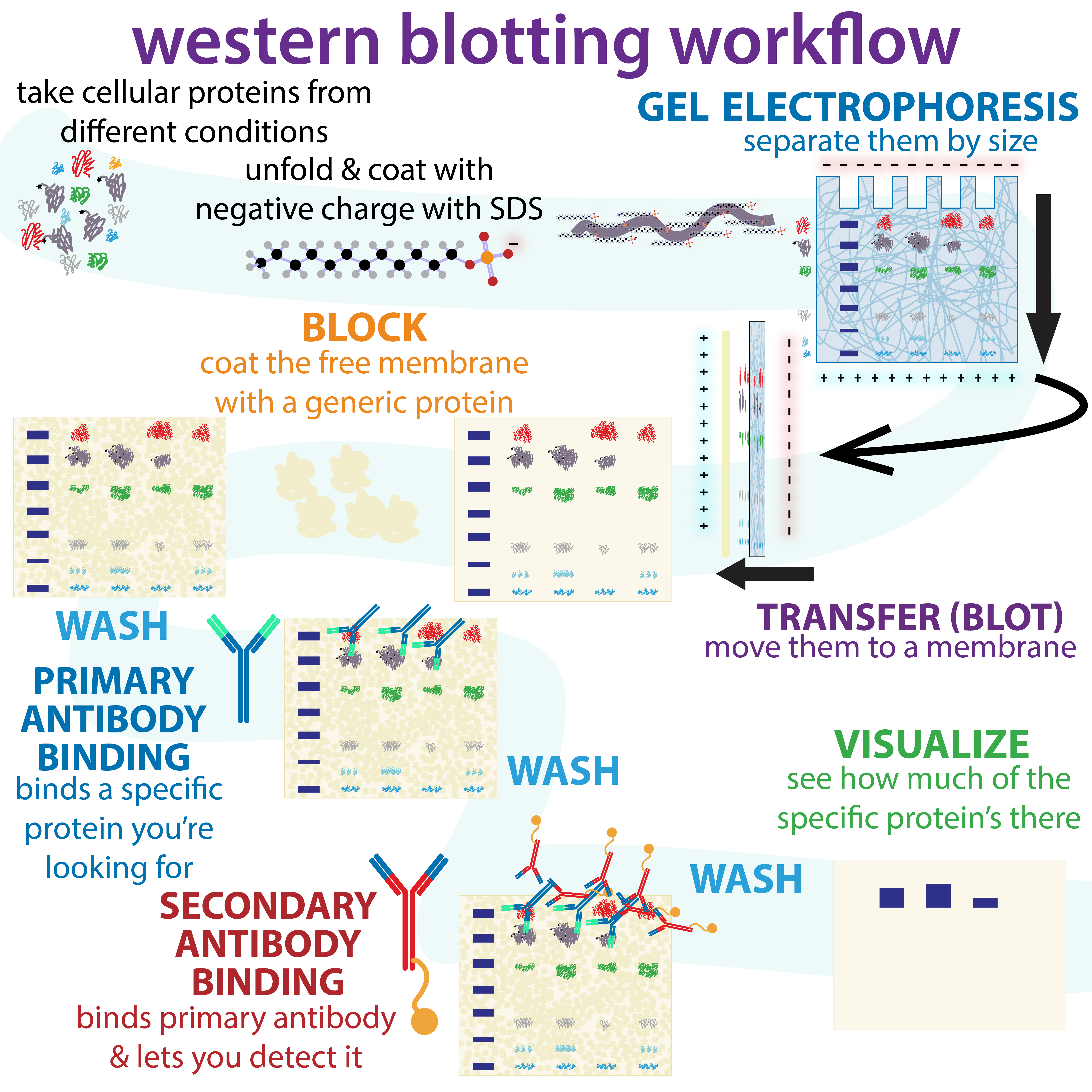
Western Blotting
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detecting the proteins, this technique is also utilized to visualize, distinguish, and quantify the different proteins in a complicated protein combination. Western blot technique uses three elements to achieve its task of separating a specific protein from a complex: separation by size, transfer of protein to a solid support, and marking target protein using a primary and secondary antibody to visualize. A synthetic or animal-derived antibody (known as the primary antibody) is created that recognizes and binds to a specific target protein. The electrophoresis membrane is washed in a solution containing the primary antibody, before excess antibody is washed off. A secondary antibody is added which recognizes and binds to the primary antibody. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streptavidin
Streptavidin is a 66.0 (tetramer) kDa protein purified from the bacterium '' Streptomyces avidinii''. Streptavidin homo-tetramers have an extraordinarily high affinity for biotin (also known as vitamin B7 or vitamin H). With a dissociation constant (Kd) on the order of ≈10−14 mol/L, the binding of biotin to streptavidin is one of the strongest non-covalent interactions known in nature. Streptavidin is used extensively in molecular biology and bionanotechnology due to the streptavidin-biotin complex's resistance to organic solvents, denaturants (e.g. guanidinium chloride), detergents (e.g. SDS, Triton X-100), proteolytic enzymes, and extremes of temperature and pH. Structure The crystal structure of streptavidin with biotin bound was reported by two groups in 1989. The structure was solved using multi wavelength anomalous diffraction by Hendrickson et al. at Columbia University and using multiple isomorphous replacement by Weber et al. at E. I. DuPont Central Research ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteinogenic Amino Acid
Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino acids, 20 in the standard genetic code and an additional 2 ( selenocysteine and pyrrolysine) that can be incorporated by special translation mechanisms. In contrast, non-proteinogenic amino acids are amino acids that are either not incorporated into proteins (like GABA, L-DOPA, or triiodothyronine), misincorporated in place of a genetically encoded amino acid, or not produced directly and in isolation by standard cellular machinery (like hydroxyproline). The latter often results from post-translational modification of proteins. Some non-proteinogenic amino acids are incorporated into nonribosomal peptides which are synthesized by non-ribosomal peptide synthetases. Both eukaryotes and prokaryotes can incorporate selenocysteine into their ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enteropeptidase
Enteropeptidase (also called enterokinase) is an enzyme produced by cells of the duodenum and is involved in digestion in humans and other animals. Enteropeptidase converts trypsinogen (a zymogen) into its active form trypsin, resulting in the subsequent activation of pancreas, pancreatic digestive enzymes. Absence of enteropeptidase results in intestinal digestion impairment. Enteropeptidase is a serine protease () consisting of a disulfide-linked heavy-chain of 82-140 kDa that anchors enterokinase in the intestinal brush border membrane and a light-chain of 35–62 kDa that contains the catalytic subunit. Enteropeptidase is a part of the chymotrypsin-clan of serine proteases, and is structurally similar to these proteins. Historical significance Enteropeptidase was discovered by Ivan Pavlov, who was awarded the 1904 Nobel Prize in Physiology or Medicine for his studies of gastrointestinal physiology. It is the first known enzyme to activate other enzymes, and it remains a r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Factor Xa
Factor X, also known by the eponym Stuart–Prower factor, is an enzyme () of the coagulation cascade. It is a serine endopeptidase (protease group S1, PA clan). Factor X is synthesized in the liver and requires vitamin K for its synthesis. Factor X is activated, by hydrolysis, into factor Xa by both factor IX (with its cofactor, factor VIII in a complex known as ''intrinsic tenase'') and factor VII with its cofactor, tissue factor (a complex known as ''extrinsic tenase''). It is therefore the first member of the ''final common pathway'' or ''thrombin pathway''. It acts by cleaving prothrombin in two places (an arg- thr and then an arg-ile bond), which yields the active thrombin. This process is optimized when factor Xa is complexed with activated co-factor V in the prothrombinase complex. Factor Xa is inactivated by protein Z-dependent protease inhibitor (ZPI), a serine protease inhibitor (serpin). The affinity of this protein for factor Xa is increased 1000-fold by the pres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thrombin
Thrombin (, ''fibrinogenase'', ''thrombase'', ''thrombofort'', ''topical'', ''thrombin-C'', ''tropostasin'', ''activated blood-coagulation factor II'', ''blood-coagulation factor IIa'', ''factor IIa'', ''E thrombin'', ''beta-thrombin'', ''gamma-thrombin'') is a serine protease, an enzyme that, in humans, is encoded by the ''F2'' gene. Prothrombin (coagulation factor II) is proteolytically cleaved to form thrombin in the clotting process. Thrombin in turn acts as a serine protease that converts soluble fibrinogen into insoluble strands of fibrin, as well as catalyzing many other coagulation-related reactions. History After the description of fibrinogen and fibrin, Alexander Schmidt hypothesised the existence of an enzyme that converts fibrinogen into fibrin in 1872. Prothrombin was discovered by Pekelharing in 1894. Physiology Synthesis Thrombin is produced by the enzymatic cleavage of two sites on prothrombin by activated Factor X (Xa). The activity of factor Xa is greatly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FlAsH-EDT2
FlAsH-EDT2 is an organoarsenic compound with molecular formula C24H18As2O5S4. Its structure is based around a fluorescein core with two 1,3,2-dithiarsolane substituents. It is used in bioanalytical research as a fluorescent label for visualising proteins in living cells. FlAsH-EDT2 is an abbreviation for fluorescin arsenical hairpin binder- ethanedithiol, and is a pale yellow or pinkish fluorogenic solid. It has a semi-structural formula (C2H4AsS2)2-(C13H5O3)-C6H4COOH, representing the dithiarsolane substituents bound to the hydroxyxanthone core, attached to an ''o''-substituted molecule of benzoic acid. FlAsH-EDT2 is used for site-specific labelling, selectively binding to proteins containing the tetracysteine (TC) motif Cys-Cys-Xxx-Xxx-Cys-Cys and becoming fluorescent when bound. It displays non-specific binding to endogenous cysteine-rich proteins, meaning it binds to sites other than the one of interest (CCXXCC). Further optimization of the TC motif has revealed improve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SpyCatcher
''Spycatcher: The Candid Autobiography of a Senior Intelligence Officer'' (1987) is a memoir written by Peter Wright, former MI5 officer and Assistant Director, and co-author Paul Greengrass. He drew on his own experiences and research into the history of the British intelligence community. Published first in Australia, the book was banned in England (but not Scotland) due to its allegations about government policy and incidents. These efforts ensured the book's notoriety, and it earned considerable profit for Wright. In 2021, the Cabinet Office was still blocking freedom of information requests for files on the ''Spycatcher'' affair despite the rule that documents should be released after 30 years. Content In ''Spycatcher'', Wright says that one of his assignments was to unmask a Soviet mole in MI5, who he says was Roger Hollis, a former MI5 Director General. His book also discusses other candidates who may have or may not have been the mole. He explores the history o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biotinylation
In biochemistry, biotinylation is the process of covalently attaching biotin to a protein, nucleic acid or other molecule. Biotinylation is rapid, specific and is unlikely to disturb the natural function of the molecule due to the small size of biotin (MW = 244.31 g/mol). Biotin binds to streptavidin and avidin with an extremely high affinity, fast on-rate, and high specificity, and these interactions are exploited in many areas of biotechnology to isolate biotinylated molecules of interest. Biotin-binding to streptavidin and avidin is resistant to extremes of heat, pH and proteolysis, making capture of biotinylated molecules possible in a wide variety of environments. Also, multiple biotin molecules can be conjugated to a protein of interest, which allows binding of multiple streptavidin, avidin or neutravidin protein molecules and increases the sensitivity of detection of the protein of interest. There is a large number of biotinylation reagents available that exploit t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |