|
Wyartite
Wyartite ·7H2O is a uranium bearing mineral named after Jean Wyart (1902–1992), mineralogist at the Sorbonne, Paris. It has greenish-black, black, or violet-black, translucent to opaque orthorhombic crystals. A hardness of 3 - 4 Mohs. Its other names are Ianthinite (of Bignand), Wyartit and Wyartita. It belongs to the uranium carbonate group of minerals. It is found next to rutherfordine in Shinkolobwe, Shaba, Zaire. Determination of the structure of wyartite provided the first evidence for a pentavalent uranium mineral. Like all uranium minerals it is radioactive Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi .... References Uranium minerals Calcium minerals Carbonate minerals Orthorhombic minerals Minerals in space group 19 Uranium(V) compounds Uranyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate Minerals
Carbonate minerals are those minerals containing the carbonate ion, . Carbonate divisions Anhydrous carbonates *Calcite group: trigonal **Calcite CaCO3 **Gaspéite (Ni,Mg,Fe2+)CO3 **Magnesite MgCO3 **Otavite CdCO3 **Rhodochrosite MnCO3 **Siderite FeCO3 **Smithsonite ZnCO3 **Spherocobaltite CoCO3 *Aragonite group: orthorhombic **Aragonite CaCO3 **Cerussite PbCO3 **Strontianite SrCO3 **Witherite BaCO3 **Rutherfordine UO2CO3 **Natrite Na2CO3 Anhydrous carbonates with compound formulas *Dolomite group: trigonal **Ankerite CaFe(CO3)2 **Dolomite (mineral), Dolomite CaMg(CO3)2 **Huntite Mg3Ca(CO3)4 **Minrecordite CaZn(CO3)2 **Barytocalcite BaCa(CO3)2 Carbonates with hydroxyl or halogen *Carbonate with hydroxide: monoclinic **Azurite Cu3(CO3)2(OH)2 **Hydrocerussite Pb3(CO3)2(OH)2 **Malachite Cu2CO3(OH)2 **Rosasite (Cu,Zn)2CO3(OH)2 **Phosgenite Pb2(CO3)Cl2 **Hydrozincite Zn5(CO3)2(OH)6 **Aurichalcite (Zn,Cu)5(CO3)2(OH)6 Hydrated carbonates *Hydromagnesite Mg5(CO3)4(OH)2.4H2O *Ikaite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
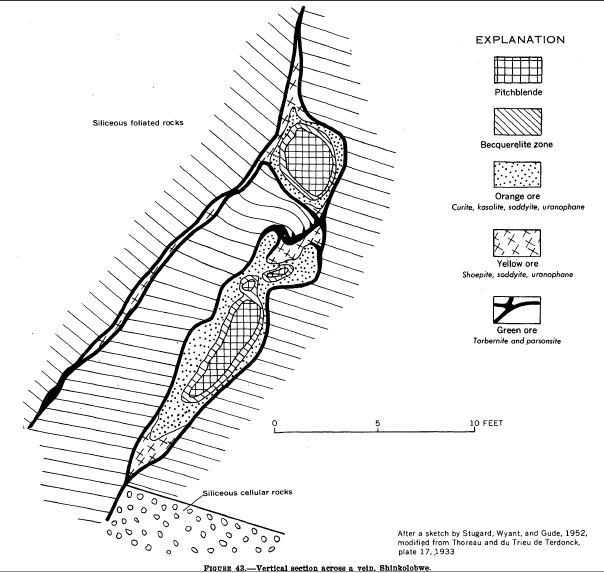
Shinkolobwe
Shinkolobwe, or Kasolo, or Chinkolobew, or Shainkolobwe, was a radium and uranium mine in the Haut-Katanga Province of the Democratic Republic of the Congo (DRC), located 20 km west of Likasi (formerly Jadotville), 20 km south of Kambove, and about 145 km northwest of Lubumbashi. The mine produced the most economical uranium ore in the world and was used for the Manhattan Project and subsequent nuclear weapons produced by the United States in the 1940s and 50s. Before World War II, uranium extracted here was originally taken to Belgium to be processed; this supply was captured by the Wehrmacht in 1940 and subsequently used for the unsuccessful German nuclear program. The Shinkolobwe mine was officially closed in 2004. Toponym The mine's name was taken from the long-gone nearby village of Shinkolobwe, which is the indigenous thorny fruit in the Lingala language. It is also slang for "a man who is easygoing on the surface but who becomes angry when provoked". ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium Carbonate
Uranyl carbonate refers to the inorganic compound with the formula UO2CO3. Also known by its mineral name rutherfordine, this material consists of uranyl (UO22+) and carbonate (CO32-). Like most uranyl salts, the compound is a polymeric, each uranium(VI) center being bonded to eight O atoms. Hydrolysis products of rutherfordine are also found in both the mineral and organic fractions of coal and its fly ash and is the main component of uranium in mine tailing seepage water. Uranyl carbonates as a class of materials Many uranyl carbonates exist, rutherfordine being the simplest stoichiometry. Most uranyl carbonates additional components including water and diverse anions and cations. A common method for concentrating uranium from a solution uses solutions of uranyl carbonates, which are passed through a resin bed where the complex ions are transferred to the resin by ion exchange with a negative ion like chloride. After build-up of the uranium complex on the resin, the uranium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Minerals In Space Group 19
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Minerals'; p. 1. In the series ''Geology: Landforms, Minerals, and Rocks''. Rosen Publishing Group. The geological definition of mineral normally excludes compounds that occur only in living organisms. However, some minerals are often biogenic (such as calcite) or are organic compounds in the sense of chemistry (such as mellite). Moreover, living organisms often synthesize inorganic minerals (such as hydroxylapatite) that also occur in rocks. The concept of mineral is distinct from rock, which is any bulk solid geologic material that is relatively homogeneous at a large enough scale. A rock may consist of one type of mineral, or may be an aggregate of two or more different types of minerals, spacially segregated into distinct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthorhombic Minerals
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a rectangular base (''a'' by ''b'') and height (''c''), such that ''a'', ''b'', and ''c'' are distinct. All three bases intersect at 90° angles, so the three lattice vectors remain mutually orthogonal. Bravais lattices There are four orthorhombic Bravais lattices: primitive orthorhombic, base-centered orthorhombic, body-centered orthorhombic, and face-centered orthorhombic. For the base-centered orthorhombic lattice, the primitive cell has the shape of a right rhombic prism;See , row oC, column Primitive, where the cell parameters are given as a1 = a2, α = β = 90° it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. Note that the length a of the primi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Minerals
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' " lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharmaceu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium Minerals
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weakly radioactive because all isotopes of uranium are unstable; the half-lives of its naturally occurring isotopes range between 159,200 years and 4.5 billion years. The most common isotopes in natural uranium are uranium-238 (which has 146 neutrons and accounts for over 99% of uranium on Earth) and uranium-235 (which has 143 neutrons). Uranium has the highest atomic weight of the primordially occurring elements. Its density is about 70% higher than that of lead, and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite. In nature, uranium is found as uranium-238 (99.2739� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered radioactive. Three of the most common types of decay are alpha decay ( ), beta decay ( ), and gamma decay ( ), all of which involve emitting one or more particles. The weak force is the mechanism that is responsible for beta decay, while the other two are governed by the electromagnetism and nuclear force. A fourth type of common decay is electron capture, in which an unstable nucleus captures an inner electron from one of the electron shells. The loss of that electron from the shell results in a cascade of electrons dropping down to that lower shell resulting in emission of discrete X-rays from the transitions. A common example is iodine-125 commonly used in medical settings. Radioactive decay is a stochastic (i.e. random) pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentavalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Description The combining capacity, or affinity of an atom of a given element is determined by the number of hydrogen atoms that it combines with. In methane, carbon has a valence of 4; in ammonia, nitrogen has a valence of 3; in water, oxygen has a valence of 2; and in hydrogen chloride, chlorine has a valence of 1. Chlorine, as it has a valence of one, can be substituted for hydrogen. Phosphorus has a valence of 5 in phosphorus pentachloride, . Valence diagrams of a compound represent the connectivity of the elements, with lines drawn between two elements, sometimes called bonds, representing a saturated valency for each element. The two tables below show some examples of different compounds, their valence diagrams, and the valences for each element of the compound. Modern definitions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zaire
Zaire (, ), officially the Republic of Zaire (french: République du Zaïre, link=no, ), was a Congolese state from 1971 to 1997 in Central Africa that was previously and is now again known as the Democratic Republic of the Congo. Zaire was, by area, the third-largest country in Africa (after Sudan and Algeria), and the 11th-largest country in the world. With a population of over 23 million inhabitants, Zaire was the most-populous officially Francophone country in Africa, as well as one of the most populous in Africa. The country was a one-party totalitarian military dictatorship, run by Mobutu Sese Seko and his ruling Popular Movement of the Revolution party. Zaire was established following Mobutu's seizure of power in a military coup in 1965, following five years of political upheaval following independence from Belgium known as the Congo Crisis. Zaire had a strongly centralist constitution, and foreign assets were nationalized. The period is sometimes referr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Katanga Province
Katanga was one of the four large provinces created in the Belgian Congo in 1914. It was one of the eleven provinces of the Democratic Republic of the Congo between 1966 and 2015, when it was split into the Tanganyika, Haut-Lomami, Lualaba, and Haut-Katanga provinces. Between 1971 and 1997 (during the rule of Mobutu Sese Seko when Congo was known as Zaire), its official name was Shaba Province. Katanga's area encompassed . Farming and ranching are carried out on the Katanga Plateau. The eastern part of the province is considered to be a rich mining region, which supplies cobalt, copper, tin, radium, uranium, and diamonds. The region's former capital, Lubumbashi, is the second-largest city in the Congo. History Copper mining in Katanga dates back over 1,000 years, and mines in the region were producing standard-sized ingots of copper for international transport by the end of the 10th century CE. In the 1890s, the province was beleaguered from the south by Cecil Rhodes' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rutherfordine
Rutherfordine is a mineral containing almost pure uranyl carbonate ( U O2 CO3). It crystallizes in the orthorhombic system in translucent lathlike, elongated, commonly radiating in fibrous, and in pulverulent, earthy to very fine-grained dense masses. It has a specific gravity of 5.7 and exhibits two directions of cleavage. It appears as brownish, brownish yellow, white, light brown orange, or light yellow fluorescent encrustations. It is also known as ''diderichite.'' It was first described in 1906 for an occurrence in the Morogoro Region of Tanzania. It was named for Ernest Rutherford. It has been reported in the Democratic Republic of Congo, the Northern Territory of Australia and a variety of locations worldwide. It occurs as a secondary mineral as a weathering product of uraninite. In addition to uraninite it occurs associated with the rare minerals becquerelite, masuyite, schoepite, kasolite, curite, boltwoodite, vandendriesscheite, billietite, metatorbernite, fourma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |