|
Vibronic
Vibronic coupling (also called nonadiabatic coupling or derivative coupling) in a molecule involves the interaction between electronic and nuclear vibrational motion. The term "vibronic" originates from the combination of the terms "vibrational" and "electronic", denoting the idea that in a molecule, vibrational and electronic interactions are interrelated and influence each other. The magnitude of vibronic coupling reflects the degree of such interrelation. In theoretical chemistry, the vibronic coupling is neglected within the Born–Oppenheimer approximation. Vibronic couplings are crucial to the understanding of nonadiabatic processes, especially near points of conical intersections. The direct calculation of vibronic couplings is not common due to difficulties associated with its evaluation. Definition Vibronic coupling describes the mixing of different electronic states as a result of small vibrations. : \mathbf_\equiv\langle\,\chi_(\mathbf;\mathbf)\,, \, \hat_\mat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Born–Huang Approximation
The Born–Huang approximation (named after Max Born and Huang Kun) is an approximation closely related to the Born–Oppenheimer approximation. It takes into account diagonal nonadiabatic effects in the electronic Hamiltonian (quantum mechanics), Hamiltonian better than the Born–Oppenheimer approximation. Despite the addition of correction terms, the electronic states remain uncoupled under the Born–Huang approximation, making it an adiabatic approximation. Shape The Born–Huang approximation asserts that the representation matrix of nuclear kinetic energy operator in the basis of Born–Oppenheimer electronic wavefunctions is diagonal: : \langle\chi_(\mathbf; \mathbf) , T_\mathrm , \chi_k(\mathbf; \mathbf)\rangle_ = \mathcal_\mathrm(\mathbf)\delta_. Consequences The Born–Huang approximation loosens the Born–Oppenheimer approximation by including some electronic matrix elements, while at the same time maintains its diagonal structure in the nuclear equatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Chemistry
Quantum chemistry, also called molecular quantum mechanics, is a branch of physical chemistry focused on the application of quantum mechanics to chemical systems, particularly towards the quantum-mechanical calculation of electronic contributions to physical and chemical properties of Molecule, molecules, Material, materials, and solutions at the atomic level. These calculations include systematically applied approximations intended to make calculations computationally feasible while still capturing as much information about important contributions to the computed Wave function, wave functions as well as to observable properties such as structures, spectra, and thermodynamic properties. Quantum chemistry is also concerned with the computation of quantum effects on molecular dynamics and chemical kinetics. Chemists rely heavily on spectroscopy through which information regarding the Quantization (physics), quantization of energy on a molecular scale can be obtained. Common metho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conical Intersection
In quantum chemistry, a conical intersection of two or more potential energy surfaces is the set of molecular geometry points where the potential energy surfaces are degenerate (intersect) and the non-adiabatic couplings between these states are non-vanishing. In the vicinity of conical intersections, the Born–Oppenheimer approximation breaks down and the coupling between electronic and nuclear motion becomes important, allowing non-adiabatic processes to take place. The location and characterization of conical intersections are therefore essential to the understanding of a wide range of important phenomena governed by non-adiabatic events, such as photoisomerization, photosynthesis, vision and the photostability of DNA. The conical intersection involving the ground electronic state potential energy surface of the C6H3F3+ molecular ion is discussed in connection with the Jahn–Teller effect in Section 13.4.2 on pages 380-388 of the textbook by Bunker and Jensen. Conical inters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Avoided Crossing
In quantum physics and quantum chemistry, an avoided crossing (sometimes called intended crossing, ''non-crossing'' or anticrossing) is the phenomenon where two eigenvalues of an Hermitian matrix representing a quantum observable and depending on ''N'' continuous real parameters cannot become equal in value ("cross") except on a manifold of ''N''-2 dimensions. The phenomenon is also known as the von Neumann–Wigner theorem. In the case of a diatomic molecule (with one parameter, namely the bond length), this means that the eigenvalues cannot cross at all. In the case of a triatomic molecule, this means that the eigenvalues can coincide only at a single point (see conical intersection). This is particularly important in quantum chemistry. In the Born–Oppenheimer approximation, the electronic molecular Hamiltonian is diagonalized on a set of distinct molecular geometries (the obtained eigenvalues are the values of the adiabatic potential energy surfaces). The geometries for which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Academic Press
Academic Press (AP) is an academic book publisher founded in 1941. It was acquired by Harcourt, Brace & World in 1969. Reed Elsevier bought Harcourt in 2000, and Academic Press is now an imprint of Elsevier. Academic Press publishes reference books, serials and online products in the subject areas of: * Communications engineering * Economics * Environmental science * Finance * Food science and nutrition * Geophysics * Life sciences * Mathematics and statistics * Neuroscience * Physical sciences * Psychology Well-known products include the ''Methods in Enzymology'' series and encyclopedias such as ''The International Encyclopedia of Public Health'' and the ''Encyclopedia of Neuroscience''. See also * Akademische Verlagsgesellschaft (AVG) — the German predecessor, founded in 1906 by Leo Jolowicz (1868–1940), the father of Walter Jolowicz Walter may refer to: People * Walter (name), both a surname and a given name * Little Walter, American blues harmonica player Marion Wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter waves and acoustic waves can also be considered forms of radiative energy, and recently gravitational waves have been associated with a spectral signature in the context of the Laser Interferometer Gravitational-Wave Observatory (LIGO) In simpler terms, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum. Historically, spectroscopy originated as the study of the wavelength dependence of the absorption by gas phase matter of visible light dispersed by a prism. Spectroscopy, primarily in the electromagnetic spectrum, is a fundamental exploratory tool in the fields of astronomy, chemistry, materials science, and physics, allowing the composition, physical structure and e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
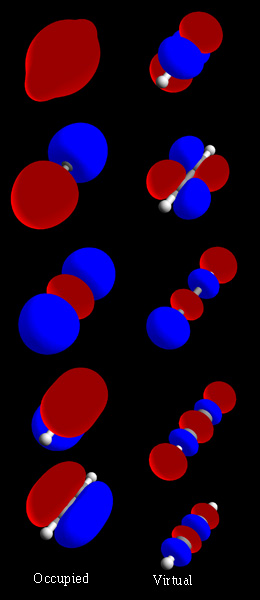
Molecular Orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The terms ''atomic orbital'' and ''molecular orbital'' were introduced by Robert S. Mulliken in 1932 to mean ''one-electron orbital wave functions''. At an elementary level, they are used to describe the ''region'' of space in which a function has a significant amplitude. In an isolated atom, the orbital electrons' location is determined by functions called atomic orbitals. When multiple atoms combine chemically into a molecule, the electrons' locations are determined by the molecule as a whole, so the atomic orbitals combine to form molecular orbitals. The electrons from the constituent atoms occupy the molecular orbitals. Mathematically, molecular orbitals are an approximate solution to the Schrödin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maria Goeppert-Mayer
Maria Goeppert Mayer (; June 28, 1906 – February 20, 1972) was a German-born American theoretical physicist, and Nobel laureate in Physics for proposing the nuclear shell model of the atomic nucleus The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron i .... She was the second woman to win a Nobel Prize in physics, the first being Marie Curie. In 1986, the Maria Goeppert-Mayer Award for early-career women physicists was established in her honor. A graduate of the University of Göttingen, Goeppert Mayer wrote her doctoral thesis on the theory of possible two-photon absorption by atoms. At the time, the chances of experimentally verifying her thesis seemed remote, but the development of the laser in the 1960s later permitted this. Today, the unit for the two-photon absorption cross sect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structure, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. History Discovery The word "''benzene''" derives from "''gum benzoin''" (benzoin res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Excited State
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to an increase in energy level above a chosen starting point, usually the ground state, but sometimes an already excited state. The temperature of a group of particles is indicative of the level of excitation (with the notable exception of systems that exhibit negative temperature). The lifetime of a system in an excited state is usually short: spontaneous or induced emission of a quantum of energy (such as a photon or a phonon) usually occurs shortly after the system is promoted to the excited state, returning the system to a state with lower energy (a less excited state or the ground state). This return to a lower energy level is often loosely described as decay and is the inverse of excitation. Long-lived excited states are often called ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Empirical Method
Empirical research is research using empirical evidence. It is also a way of gaining knowledge by means of direct and indirect observation or experience. Empiricism values some research more than other kinds. Empirical evidence (the record of one's direct observations or experiences) can be analyzed quantitatively or qualitatively. Quantifying the evidence or making sense of it in qualitative form, a researcher can answer empirical questions, which should be clearly defined and answerable with the evidence collected (usually called data). Research design varies by field and by the question being investigated. Many researchers combine qualitative and quantitative forms of analysis to better answer questions that cannot be studied in laboratory settings, particularly in the social sciences and in education. In some fields, quantitative research may begin with a research question (e.g., "Does listening to vocal music during the learning of a word list have an effect on later memory ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



2.png)