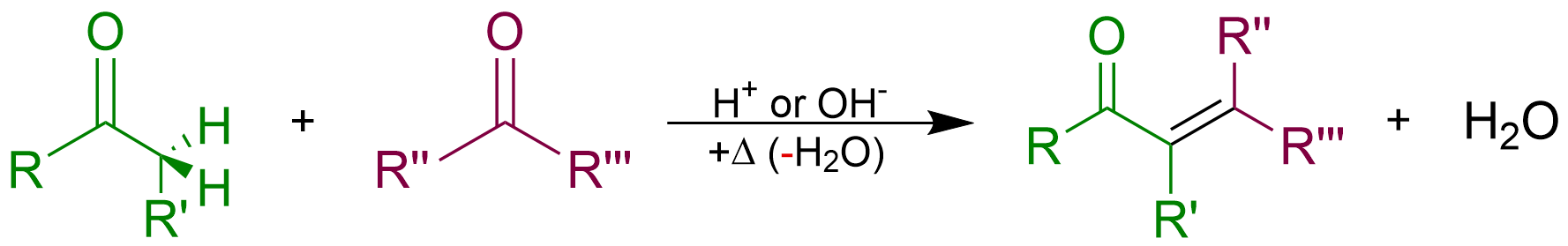|
Valeraldehyde
Pentanal (also called valeraldehyde) is the organic compound is an alkyl aldehyde, molecular formula C5H10O. It is used in flavorings, resin chemistry, and rubber accelerators. Its smell is described as fermented, bready, fruity, nutty, berry. Production Pentanal is obtained by hydroformylation of butene. Also C4 mixtures can be used as starting material like the so-called raffinate II, which is produced by steam cracking and contains (''Z'')- and (''E'')-2-butene, 1-butene, butane and isobutane. The conversion to the product is accomplished with synthesis gas in the presence of a catalyst consisting of a rhodium-bisphosphite complex and a sterically hindered secondary amine with a selectivity toward pentanal of at least 90%. Use Pentanal is used ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valeric Acid
Valeric acid or pentanoic acid is a straight-chain alkyl carboxylic acid with the chemical formula . Like other low-molecular-weight carboxylic acids, it has an unpleasant odor. It is found in the perennial flowering plant ''Valeriana officinalis'', from which it gets its name. Its primary use is in the synthesis of its esters. Salts and esters of valeric acid are known as valerates or pentanoates. Volatile esters of valeric acid tend to have pleasant odors and are used in perfumes and cosmetics. Several, including ethyl valerate and pentyl valerate are used as food additives because of their fruity flavors. History Valeric acid is a minor constituent of the perennial flowering plant valerian (''Valeriana officinalis''), from which it gets its name. The dried root of this plant has been used medicinally since antiquity. The related isovaleric acid shares its unpleasant odor and their chemical identity was investigated by oxidation of the components of fusel alcohol, which in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres. Structure and bonding Aldehydes feature a carbon center that is connected by a double bond to oxygen and a single bond to hydrogen and single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The C=O bond length is about 120-122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile aldehydes have pungent odors. Al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sterically Hindered
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel. Steric hindrance Steric hindrance is a consequence of steric effects. Steric hindrance is the slowing of chemical reactions due to steric bulk. It is usually manifested in ''intermolecular reactions'', whereas discussion of steric effects often focus on ''intramolecular interactions''. Steric hindrance is often exploited to control selectivity, such as slowing unwanted side-reactions. Steric hindrance between adjacent groups can also affect torsional ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DPHP
Di(2-propylheptyl) phthalate (also known as bis(2-propylheptyl) benzene-1,2-dicarboxylate, di(propylheptyl) orthophthalate, or DPHP) is an organic compound with the formula C28H46O4. It is a phthalate and is the diester of phthalic acid and the 10-carbon branched-chain alcohol 2-propylheptanol. This colorless, viscous liquid is used for softening PVC plastics and is a general purpose PVC plasticizer. It possesses very good plasticizing properties and may be used as a direct replacement for DEHP and DINP Diisononyl phthalate (DINP) is a phthalate used as a plasticizer. DINP is typically a mixture of chemical compounds consisting of various isononyl esters of phthalic acid, and is commonly used in a large variety of plastic items. Health Issues ... in many applications. References Phthalate esters {{ester-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasticizer
A plasticizer ( UK: plasticiser) is a substance that is added to a material to make it softer and more flexible, to increase its plasticity, to decrease its viscosity, and/or to decrease friction during its handling in manufacture. Plasticizers are commonly added to polymers such as plastics and rubber, either to facilitate the handling of the raw material during fabrication, or to meet the demands of the end product's application. For example, plasticizers are commonly added to polyvinyl chloride (PVC), which otherwise is hard and brittle, to make it soft and pliable; which makes it suitable for products such as shower curtains, vinyl flooring, clothing, bags, flexible plastic tubing, and electric wire insulation/coating. Plasticizers are also often added to concrete formulations to make them more workable and fluid for pouring, thus allowing the water contents to be reduced. Similarly, they are often added to clays, stucco, solid rocket fuel, and other pastes prior t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-propylheptanol
2-Propylheptanol (2PH) is a colourless waxy or oily solid. Production 2-Propylheptanol is an oxo alcohol, meaning that it is produced from the hydroformylation ("oxo synthesis") of C4 alkenes followed by hydrogenation of the resulting aldehyde. The production route is similar to that for 2-Ethylhexanol 2-Ethylhexanol (abbreviated 2-EH) is an organic compound with formula CHO. It is a branched, eight-carbon chiral alcohol (chemistry), alcohol. It is a colorless liquid that is poorly soluble in water but soluble in most organic solvents. It is prod .... Applications Such compounds enjoy many applications, including as raw materials for plasticizers, resins, processing solvents, and precursors to detergents. Heat stabilizers manufactured for PVC compounds use similar high boiling and high molecular weight oxo-alcohols, which enhance product performance. A further application area of this C10 alcohol is for the manufacture of oleate- and palmitate-based materials used by the cosmeti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldol Condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration to give a conjugated enone. The overall reaction is as follows (where the Rs can be H): Aldol condensations are important in organic synthesis and biochemistry as ways to form carbon–carbon bonds. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or "aldol" (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals. The term ''aldol condensation'' is also commonly used, especially in biochemistry, to refer to just the first (addition) stage of the process—the aldol reaction itself—as catalyzed by aldolases. However, this is formally an addition reaction rather than a condensation reaction because it does not invo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vulcanization
Vulcanization (British: Vulcanisation) is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to include the hardening of other (synthetic) rubbers via various means. Examples include silicone rubber via room temperature vulcanizing and chloroprene rubber (neoprene) using metal oxides. Vulcanization can be defined as the curing of elastomers, with the terms 'vulcanization' and 'curing' sometimes used interchangeably in this context. It works by forming cross-links between sections of polymer chain which results in increased rigidity and durability, as well as other changes in the mechanical and electrical properties of the material. Vulcanization, in common with the curing of other thermosetting polymers, is generally irreversible. The word vulcanization is derived from Vulcan, the Roman god of fire and forge. History Rubber—latex� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavoring
A flavoring (or flavouring), also known as flavor (or flavour) or flavorant, is a food additive used to improve the taste or smell of food. It changes the perceptual impression of food as determined primarily by the chemoreceptors of the gustatory and olfactory systems. Along with additives, other components like sugars determine the taste of food. A flavoring is defined as a substance that gives another substance taste, altering the characteristics of the solute, causing it to become sweet, sour, tangy, etc. Although the term, in common language, denotes the combined chemical sensations of taste and smell, the same term is used in the fragrance and flavors industry to refer to edible chemicals and extracts that alter the flavor of food and food products through the sense of smell. Owing to the high cost, or unavailability of natural flavor extracts, most commercial flavorings are "nature-identical", which means that they are the chemical equivalent of natural flavors, but ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactivity–selectivity Principle
In chemistry the reactivity–selectivity principle or RSP states that a more reactive chemical compound or reactive intermediate is less selective in chemical reactions. In this context selectivity represents the ratio of reaction rates. This principle was generally accepted until the 1970s when too many exceptions started to appear. The principle is now considered obsolete. A classic example of perceived RSP found in older organic chemistry textbooks concerns the free radical halogenation of simple alkanes. Whereas the relatively unreactive bromine reacts with 2-methylbutane predominantly to 2-bromo-2-methylbutane, the reaction with much more reactive chlorine results in a mixture of all four regioisomers. Another example of RSP can be found in the selectivity of the reaction of certain carbocations with azides and water. The very stable triphenylmethyl carbocation derived from solvolysis of the corresponding triphenylmethyl chloride reacts 100 times faster with the azide anion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |