|
Unfolded State
In biochemistry, denaturation is a process in which proteins or nucleic acids lose Protein structure, folded structure present in their native state due to various factors, including application of some external stress or compound, such as a strong acid or base (chemistry), base, a concentrated inorganic salt, an organic compound, organic solvent (e.g., Alcohol (chemistry), alcohol or chloroform), agitation, radiation, or heat. If proteins in a living Cell (biology), cell are denatured, this results in disruption of cell activity and possibly Apoptosis, cell death. Protein denaturation is also a consequence of cell death. Denatured proteins can exhibit a wide range of characteristics, from conformational change and loss of solubility or dissociation of Cofactor (biochemistry), cofactors to Protein aggregation, aggregation due to the exposure of hydrophobic groups. The loss of solubility as a result of denaturation is called Precipitation (chemistry), ''coagulation''. Denatured pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Q10 Graphs
Q1 or Q-1 may refer to: Transport Air * Radioplane Q-1, an American experimental unmanned aircraft of the 1950s * The primary United States Air Force designation for a series of unmanned aerial vehicles built by General Atomics, which includes the MQ-1 Predator and the MQ-1C Warrior Road * Q1 (New York City bus) * Rossion Q1, a sports car from US car maker 1g Racing/Rossion Automotive Rail * LNER Thompson Class Q1, a class of steam locomotives of the London and North Eastern Railway, UK * PRR Q1, a steam locomotive of the Pennsylvania Railroad, USA * SECR Q1 class, a steam locomotive of the South Eastern and Chatham Railway, UK * SR Q1 class, a steam locomotive of the Southern Railway, UK Science and technology * First quartile in descriptive statistics * DIGITAL Q1, a digital camera model (Fujifilm) * Samsung Q1, an Ultra Mobile Personal Computer (UMPC) * Q1 microcomputer, an early offering in the History of personal computers#Q1, history of personal computers * The Un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elsevier
Elsevier ( ) is a Dutch academic publishing company specializing in scientific, technical, and medical content. Its products include journals such as ''The Lancet'', ''Cell (journal), Cell'', the ScienceDirect collection of electronic journals, ''Trends (journals), Trends'', the ''Current Opinion (Elsevier), Current Opinion'' series, the online citation database Scopus, the SciVal tool for measuring research performance, the ClinicalKey search engine for clinicians, and the ClinicalPath evidence-based cancer care service. Elsevier's products and services include digital tools for Data management platform, data management, instruction, research analytics, and assessment. Elsevier is part of the RELX Group, known until 2015 as Reed Elsevier, a publicly traded company. According to RELX reports, in 2022 Elsevier published more than 600,000 articles annually in over 2,800 journals. As of 2018, its archives contained over 17 million documents and 40,000 Ebook, e-books, with over one b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently bonded to a more Electronegativity, electronegative donor atom or group (Dn), interacts with another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Unlike simple Dipole–dipole attraction, dipole–dipole interactions, hydrogen bonding arises from charge transfer (nB → σ*AH), Atomic orbital, orbital interactions, and quantum mechanical Delocalized electron, delocalization, making it a resonance-assisted interaction rather than a mere electrostatic attraction. The general notation for hydrogen bonding is Dn−H···Ac, where the solid line represents a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are nitrogen (N), oxyg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
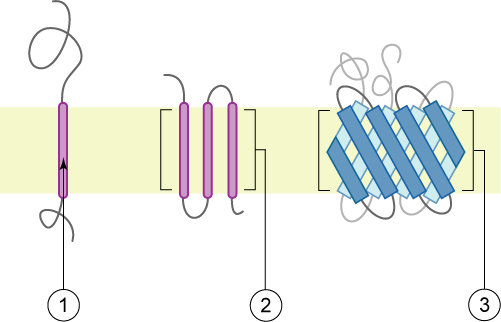
Membrane Protein
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane and can either penetrate the membrane (Transmembrane protein, transmembrane) or associate with one or the other side of a membrane (Integral monotopic protein, integral monotopic). Peripheral membrane proteins are transiently associated with the cell membrane. Membrane proteins are common, and medically important—about a third of all human proteins are membrane proteins, and these are targets for more than half of all drugs. Nonetheless, compared to other classes of proteins, determining membrane protein structures remains a challenge in large part due to the difficulty in establishing experimental conditions that can preserve the correct (Native state, native) Protein structure, conformation of the protein in isolation from its native ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Globular Protein
In biochemistry, globular proteins or spheroproteins are spherical ("globe-like") proteins and are one of the common protein types (the others being fibrous, disordered and membrane proteins). Globular proteins are somewhat water-soluble (forming colloids in water), unlike the fibrous or membrane proteins. There are multiple fold classes of globular proteins, since there are many different architectures that can fold into a roughly spherical shape. The term globin can refer more specifically to proteins including the globin fold. Globular structure and solubility The term globular protein is quite old (dating probably from the 19th century) and is now somewhat archaic given the hundreds of thousands of proteins and more elegant and descriptive structural motif vocabulary. The globular nature of these proteins can be determined without the means of modern techniques, but only by using ultracentrifuges or dynamic light scattering techniques. The spherical structure is induce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Folding
Protein folding is the physical process by which a protein, after Protein biosynthesis, synthesis by a ribosome as a linear chain of Amino acid, amino acids, changes from an unstable random coil into a more ordered protein tertiary structure, three-dimensional structure. This structure permits the protein to become biologically functional or active. The folding of many proteins begins even during the translation of the polypeptide chain. The amino acids interact with each other to produce a well-defined three-dimensional structure, known as the protein's native state. This structure is determined by the amino-acid sequence or primary structure. The correct three-dimensional structure is essential to function, although some parts of functional proteins Intrinsically unstructured proteins, may remain unfolded, indicating that protein dynamics are important. Failure to fold into a native structure generally produces inactive proteins, but in some instances, misfolded proteins have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metalloenzyme
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins (out of ~20,000) contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins. Abundance It is estimated that approximately half of all proteins contain a metal. In another estimate, about one quarter to one third of all proteins are proposed to require metals to carry out their functions. Thus, metalloproteins have many different functions in cells, such as storage and transport of proteins, enzymes and signal transduction proteins, or infectious diseases. The abundance of metal binding proteins may be inherent to the amino acids that proteins use, as even artificial proteins without evolutionary history will readily bind metals. Most metals in the human body are bound to proteins. For instance, the relatively high concentration of iron in the human body ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Precipitation (chemistry)
In an aqueous solution, precipitation is the "sedimentation of a solid material (a precipitate) from a liquid solution". The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the precipitant. The clear liquid remaining above the precipitated or the centrifuged solid phase is also called the supernate or supernatant. The notion of precipitation can also be extended to other domains of chemistry (organic chemistry and biochemistry) and even be applied to the solid phases (e.g. metallurgy and alloys) when solid impurities segregation (materials science), segregate from a solid phase. Supersaturation The precipitation of a compound may occur when its concentration exceeds its solubility. This can be due to temperature changes, solvent evaporation, or by mixing solvents. Precipitation occurs more rapidly from a strongly supersaturated solution. The formation of a pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water. Hydrophobic molecules tend to be nonpolar and, thus, prefer other neutral molecules and nonpolar solvents. Because water molecules are polar, hydrophobes do not dissolve well among them. Hydrophobic molecules in water often cluster together, forming micelles. Water on hydrophobic surfaces will exhibit a high contact angle. Examples of hydrophobic molecules include the alkanes, oils, fats, and greasy substances in general. Hydrophobic materials are used for oil removal from water, the management of oil spills, and chemical separation processes to remove non-polar substances from polar compounds. The term ''hydrophobic''—which comes from the Ancient Greek (), "having a fear of water", constructed Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon. revised and augmented ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Aggregation
In molecular biology, protein aggregation is a phenomenon in which intrinsically disordered proteins, intrinsically-disordered or mis-folded proteins aggregate (i.e., accumulate and clump together) either intra- or extracellularly. Protein aggregates have been implicated in a wide variety of diseases known as amyloidoses, including Amyotrophic lateral sclerosis, ALS, Alzheimer's, Parkinson's and prion disease. After synthesis, proteins typically Protein folding, fold into a particular Protein tertiary structure, three-dimensional conformation that is the most Thermodynamic equilibrium, thermodynamically favorable: their native state. This folding process is driven by the hydrophobic effect: a tendency for hydrophobic (water-fearing) portions of the protein to shield themselves from the hydrophilic (water-loving) environment of the cell by burying into the interior of the protein. Thus, the exterior of a protein is typically hydrophilic, whereas the interior is typically hydrophob ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst (a catalyst is a substance that increases the rate of a chemical reaction). Cofactors can be considered "helper molecules" that assist in biochemical transformations. The rates at which these happen are characterized in an area of study called enzyme kinetics. Cofactors typically differ from ligands in that they often derive their function by remaining bound. Cofactors can be classified into two types: inorganic ions and complex organic molecules called coenzymes. Coenzymes are mostly derived from vitamins and other organic essential nutrients in small amounts. (Some scientists limit the use of the term "cofactor" for inorganic substances; both types are included here.) Coenzymes are further divided into two types. The first is called a " prosthetic group", which consists of a coenzyme that is tightly (or even covalently and, therefore, permanently) bound to a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubility
In chemistry, solubility is the ability of a chemical substance, substance, the solute, to form a solution (chemistry), solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a wikt:saturated#Chemistry, saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be "miscibility, miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |