|
Uncoupler
An uncoupler or uncoupling agent is a molecule that disrupts oxidative phosphorylation in prokaryotes and mitochondria or photophosphorylation in chloroplasts and cyanobacteria by dissociating the reactions of ATP synthesis from the electron transport chain. The result is that the cell or mitochondrion expends energy to generate a proton-motive force, but the proton-motive force is dissipated before the ATP synthase can recapture this energy and use it to make ATP. Uncouplers are capable of transporting protons through mitochondrial and lipid membranes. Description Classical uncouplers have five properties: # the complete release of respiratory control # the substitution of all coupled processes (ATP synthesis, transhydrogenation, reverse electron flow, active transport of cations, etc.) by a cyclic proton transport mediated by the uncoupler # the elimination of all protonic and cationic gradients generated across the mitochondrial or prokaryotic membrane # no discrimination in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BAM15
BAM15 is a novel mitochondrial protonophore uncoupler capable of protecting mammals from acute renal ischemic-reperfusion injury and cold-induced microtubule damage. References {{reflist External links Fat-fighting molecule sees the body burn more fuel Oxadiazoles Pyrazines Bicyclic compounds Fluoroarenes Anilines Secondary amines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
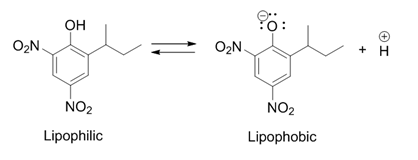
2,4-dinitrophenol
2,4-Dinitrophenol (2,4-DNP or simply DNP) is an organic compound with the formula HOC6H3(NO2)2. It is a yellow, crystalline solid that has a sweet, musty odor. It sublimates, is volatile with steam, and is soluble in most organic solvents as well as aqueous alkaline solutions. When in a dry form, it is a high explosive and has an instantaneous explosion hazard. It is a precursor to other chemicals and is biochemically active, uncoupling oxidative phosphorylation from the electron transport chain in cells with mitochondria, by allowing protons to pass from the intermembrane space into the mitochondrial matrix. Oxidative phosphorylation is a highly regulated step in aerobic respiration that is inhibited, among other factors, by normal cellular levels of ATP. Uncoupling it results in chemical energy from diet and energy stores such as triglycerides being wasted as heat with minimal regulation, leading to dangerously high body temperatures that may develop into heatstroke. Its use as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dinoseb
Dinoseb is a common industry name for 6-sec-butyl-2,4-dinitrophenol, a herbicide in the dinitrophenol family. It is a crystalline orange solid which does not readily dissolve in water. Dinoseb is banned as an herbicide in the European Union (EU) and the United States because of its toxicity. It also finds use as a polymerisation inhibitor, where it is often referred to as DNBP. It is used to prevent the thermally induced polymerisation of styrene and other unsaturated monomers when they are being purified by distillation. History In 1892, dinitro-''ortho''-cresol (2,4-dinitro-6-methylphenol), a chemical compound closely related to dinoseb, was discovered in Germany and first used as an insecticide. It was later also used as an herbicide and also fungicide after those characteristics were discovered. In 1945 the ''ortho''-methyl group was replaced by a ''sec''-butyl group, producing dinoseb. This compound had a superior contact and stomach activity on insects and mites. Dinoseb b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dinoterb
Dinoterb is a chemical compound used as an herbicide. It is an uncoupler An uncoupler or uncoupling agent is a molecule that disrupts oxidative phosphorylation in prokaryotes and mitochondria or photophosphorylation in chloroplasts and cyanobacteria by dissociating the reactions of ATP synthesis from the electron transp .... References Herbicides Dinitrophenols Tert-butyl compounds Uncoupling agents {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reverse Electron Flow
Reverse electron flow (also known as reverse electron transport) is a mechanism in microbial metabolism. Chemolithotrophs using an electron donor with a higher redox potential than NAD(P)+/NAD(P)H, such as nitrite or sulfur compounds, must use energy to reduce NAD(P)+. This energy is supplied by consuming proton motive force to drive electrons in a reverse direction through an electron transport chain and is thus the reverse process as forward electron transport. In some cases, the energy consumed in reverse electron transport is five times greater than energy gained from the forward process. Autotrophs can use this process to supply reducing power for inorganic carbon fixation. Reverse electron transfer (RET) is the process that can occur in respiring mitochondria, when a small fraction of electrons from reduced ubiquinol is driven upstream by the membrane potential towards to mitochondrial complex I. This result in reduction of oxidized pyridine nucleotide ( NAD+ or NADP+). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mitochondria
A mitochondrion (; ) is an organelle found in the Cell (biology), cells of most Eukaryotes, such as animals, plants and Fungus, fungi. Mitochondria have a double lipid bilayer, membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'' was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase coined by Philip Siekevitz in a 1957 article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). A large number of unicellular organisms, such as microsporidia, parabasalids and diplomonads, have reduced or transformed their mitochondria into mitosome, other structures. One eukaryote, ''Monocercomonoides'', is known to have completely lost its mitocho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Transport Chain
An electron transport chain (ETC) is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. The electrons that transferred from NADH and FADH2 to the ETC involves 4 multi-subunit large enzymes complexes and 2 mobile electron carriers. Many of the enzymes in the electron transport chain are membrane-bound. The flow of electrons through the electron transport chain is an exergonic process. The energy from the redox reactions creates an electrochemical proton gradient that drives the synthesis of adenosine triphosphate (ATP). In aerobic respiration, the flow of electrons terminates with molecular oxygen as the final electron acceptor. In anaerobic respiration, other electron acceptors are used, such as sulfate. In an electron transport chain, the redo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylhydrazone
Hydrazones are a class of organic compounds with the structure . They are related to ketones and aldehydes by the replacement of the oxygen =O with the = functional group. They are formed usually by the action of hydrazine on ketones or aldehydes. Synthesis Hydrazine, organohydrazines, and 1,1-diorganohydrazines react with aldehydes and ketones to give hydrazones. : Phenylhydrazine reacts with reducing sugars to form hydrazones known as osazones, which was developed by German chemist Emil Fischer as a test to differentiate monosaccharides. Uses Hydrazones are the basis for various analyses of ketones and aldehydes. For example, dinitrophenylhydrazine coated onto a silica sorbent is the basis of an adsorption cartridge. The hydrazones are then eluted and analyzed by HPLC using a UV detector. The compound carbonyl cyanide-''p''-trifluoromethoxyphenylhydrazone (abbreviated as FCCP) is used to uncouple ATP synthesis and reduction of oxygen in oxidative phosphorylation in molec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dinitro-ortho-cresol
Dinitro-''ortho''-cresol (DNOC) is an organic compound with the structural formula CH3C6H2(NO2)2OH. It is a yellow solid that is only slightly soluble in water. DNOC and some related derivatives have been used as herbicides. Preparation This compound is prepared by disulfonation of ''o''-cresol. The resulting disulfonate is then treated with nitric acid to give DNOC. A variety of related derivatives are known including those where the methyl group is replaced by ''sec''-butyl (dinoseb), ''tert''-butyl (dinoterb), and 1-methylheptyl ( dinocap). These are prepared by the direct nitration of the alkyphenols. Applications and safety This toxicant is an uncoupler, which means that it interferes with adenosine triphosphate (ATP) production. Symptoms of dinitro-''ortho''-cresol poisoning, due to ingestion or other forms of exposure, include confusion, headache, shortness of breath, and sweat Perspiration, also known as sweating, is the production of fluids secreted by the sweat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhodamine
Rhodamine is a family of related dyes, a subset of the triarylmethane dyes. They are derivatives of xanthene. Important members of the rhodamine family are Rhodamine 6G, Rhodamine 123, and Rhodamine B. They are mainly used to dye paper and inks, but they lack the lightfastness for fabric dyeing. Use Aside from their major applications, they are often used as a tracer dye, e.g. to determine the rate and direction of flow and transport of water. Rhodamine dyes fluoresce and can thus be detected easily and inexpensively with instruments called fluorometers. Rhodamine dyes are used extensively in biotechnology applications such as fluorescence microscopy, flow cytometry, fluorescence correlation spectroscopy and ELISA. Rhodamine 123 is used in biochemistry to inhibit mitochondrion function. Rhodamine 123 appears to bind to the mitochondrial membranes and inhibit transport processes, especially the electron transport chain, thus slowing down cellular respiration. It is a substra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Cyanide M-chlorophenyl Hydrazone
Carbonyl cyanide ''m''-chlorophenyl hydrazone (CCCP; also known as 3-chlorophenyl)hydrazonoalononitrile) is a chemical inhibitor of oxidative phosphorylation. It is a nitrile, hydrazone and protonophore. In general, CCCP causes the gradual destruction of living cells and death of the organism, although mild doses inducing partial decoupling have been shown to increase median and maximum lifespan in '' C. elegans'' models, suggesting a degree of hormesis. CCCP causes an uncoupling of the proton gradient that is established during the normal activity of electron carriers in the electron transport chain. The chemical acts essentially as an ionophore and reduces the ability of ATP synthase to function optimally. It is routinely used as an experimental uncoupling agent in cell and molecular biology, particularly in the study of mitophagy, where it was integral in discovering the role of the Parkinson's disease-associated ubiquitin ligase Parkin. Outside of its effects on mitoch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidative Phosphorylation
Oxidative phosphorylation (UK , US ) or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing chemical energy in order to produce adenosine triphosphate (ATP). In eukaryotes, this takes place inside mitochondria. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is so pervasive because it releases more energy than alternative fermentation processes such as anaerobic glycolysis. The energy stored in the chemical bonds of glucose is released by the cell in the citric acid cycle producing carbon dioxide, and the energetic electron donors NADH and FADH. Oxidative phosphorylation uses these molecules and O2 to produce ATP, which is used throughout the cell whenever energy is needed. During oxidative phosphorylation, electrons are transferred from the electron donors to a series of electron acceptors in a series of redox reactions ending in oxygen, who ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |