Dinoseb on:
[Wikipedia]
[Google]
[Amazon]
Dinoseb is a common industry name for 6-sec-butyl-2,4-dinitrophenol, a
 It uses this property to transport protons through the
It uses this property to transport protons through the
 After oral administration of dinoseb tagged with 14C to rats and mice, it turned out that 40 to 65% of the 14C was excreted in the urine and 30 to 40% ended up in the feces. TLC data showed the presence of different metabolites of dinoseb, although these were not identified. This finding was confirmed by different in vitro and in vivo studies.
During one study, 14C-dinoseb was administered to pregnant mice. The data showed that the rate of absorption after
After oral administration of dinoseb tagged with 14C to rats and mice, it turned out that 40 to 65% of the 14C was excreted in the urine and 30 to 40% ended up in the feces. TLC data showed the presence of different metabolites of dinoseb, although these were not identified. This finding was confirmed by different in vitro and in vivo studies.
During one study, 14C-dinoseb was administered to pregnant mice. The data showed that the rate of absorption after
 The second step in the synthesis of dinoseb is the
The second step in the synthesis of dinoseb is the  2-(1-methylpropyl)phenol takes up the
2-(1-methylpropyl)phenol takes up the  The product of this reaction can undergo a second
The product of this reaction can undergo a second 
US EPA datasheet
{{Herbicides Herbicides Dinitrophenols Uncoupling agents
herbicide
Herbicides (, ), also commonly known as weedkillers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page f ...
in the dinitrophenol
Dinitrophenols are chemical compounds which are nitro derivatives of phenol.
There are six isomers of dinitrophenol:
* 2,3-Dinitrophenol
* 2,4-Dinitrophenol
2,4-Dinitrophenol (2,4-DNP or simply DNP) is an organic compound with the formula H ...
family. It is a crystalline orange solid which does not readily dissolve in water. Dinoseb is banned as an herbicide in the European Union
The European Union (EU) is a supranational union, supranational political union, political and economic union of Member state of the European Union, member states that are located primarily in Europe, Europe. The union has a total area of ...
(EU) and the United States because of its toxicity.
It also finds use as a polymerisation inhibitor Polymerisation inhibitors (US: polymerization inhibitors) are chemical compounds added to monomers to prevent their auto-polymerisation. Unsaturated monomers such as acrylates, vinyl chloride, butadiene and styrene require inhibitors for both proces ...
, where it is often referred to as DNBP. It is used to prevent the thermally induced polymerisation of styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ...
and other unsaturated monomer
In chemistry, a monomer ( ; '' mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
s when they are being purified by distillation.
History
In 1892, dinitro-''ortho''-cresol (2,4-dinitro-6-methylphenol), a chemical compound closely related to dinoseb, was discovered in Germany and first used as aninsecticide
Insecticides are substances used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed t ...
. It was later also used as an herbicide and also fungicide
Fungicides are biocidal chemical compounds or biological organisms used to kill parasitic fungi or their spores. A fungistatic inhibits their growth. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality ...
after those characteristics were discovered. In 1945 the ''ortho''-methyl group was replaced by a ''sec''-butyl group, producing dinoseb. This compound had a superior contact and stomach activity on insects and mites. Dinoseb became commercially available in 1945 and was approved for use in the United States based on safety data from Industrial Bio-Test Laboratories. On January 13, 1984 the Danish ship Dana Optima lost 80 drums of Dinoseb during their trip from North Shields, England to Esbjerg
Esbjerg (, ) is a seaport town and seat of Esbjerg Municipality on the west coast of the Jutland peninsula in southwest Denmark. By road, it is west of Kolding and southwest of Aarhus. With an urban population of 71,698 (1 January 2022) ...
, Denmark. After four months 72 drums were found and recovered. Dinoseb was withdrawn from the market in 1986 due to an increased threat of birth defects after female field workers were exposed to the chemical. It could also cause sterility in men who were exposed to the chemical.
Uses
Dinoseb is an herbicide that was once widely used for weed-control when producing crops like soybeans, vegetables, fruits and nuts, or citrus. In the present, dinoseb is banned in the EU, and the United States due to its high toxicity. However, dinoseb is still used in China for example; evidenced by the fact that it is found in rain- and drinking water. Nowadays there are other, safer herbicides that can be used. Dinoseb was also used as aninsecticide
Insecticides are substances used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed t ...
to protect grapes. On the internet, dinoseb and other dinitrophenol
Dinitrophenols are chemical compounds which are nitro derivatives of phenol.
There are six isomers of dinitrophenol:
* 2,3-Dinitrophenol
* 2,4-Dinitrophenol
2,4-Dinitrophenol (2,4-DNP or simply DNP) is an organic compound with the formula H ...
s are bought as weight-loss pills. It is very dangerous however, and many people have died of accidental overdose.
Mechanism of action
Dinoseb is an uncoupler ofoxidative phosphorylation
Oxidative phosphorylation (UK , US ) or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing chemical energy in order to produce adenosine t ...
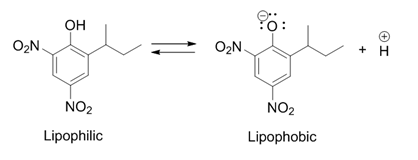
. It is a weak acid that can pass through lipid membranes when it's in the undissociated form.
 It uses this property to transport protons through the
It uses this property to transport protons through the inner mitochondrial membrane
The inner mitochondrial membrane (IMM) is the mitochondrial membrane which separates the mitochondrial matrix from the intermembrane space.
Structure
The structure of the inner mitochondrial membrane is extensively folded and compartmentalized. ...
(IMM). Protons are taken up from the intermembrane space and after transport through the IMM, they are released again in the mitochondrial matrix. Dinoseb in the dissociated form is negatively charged, which causes it to move to the intermembrane space because of the electrochemical gradient
An electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a membrane. The gradient consists of two parts, the chemical gradient, or difference in solute concentration across a membrane, and ...
that exists across the IMM.
By lowering the proton gradient, dinoseb removes the cell's ability to produce ATP, resulting in the death of the cell.
In plants, dinoseb also inhibits photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored in ...
by inhibiting the electron flow from photocomplex II to plastoquinone
Plastoquinone (PQ) is an isoprenoid quinone molecule involved in the electron transport chain in the light-dependent reactions of photosynthesis. The most common form of plastoquinone, known as PQ-A or PQ-9, is a 2,3-dimethyl-1,4-benzoquinone ...
. As a result, the plastoquinone can't create a proton gradient and no ATP is produced by the ATP synthase
ATP synthase is a protein that catalyzes the formation of the energy storage molecule adenosine triphosphate (ATP) using adenosine diphosphate (ADP) and inorganic phosphate (Pi). It is classified under ligases as it changes ADP by the formation ...
. Also, NADP can't be reduced to form NADPH, which removes the ability to create glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, u ...
from carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
. This also leads to cell death.
Metabolism and biotransformation
intraperitoneal administration
Intraperitoneal injection or IP injection is the injection of a substance into the peritoneum (body cavity). It is more often applied to animals than to humans. In general, it is preferred when large amounts of blood replacement fluids are needed ...
was much higher than after an oral administration. Furthermore, molecules containing 14C were found in all tissues of the mother and the embryo, although the embryonic tissues contained a lower concentration.
Three hours after oral or intraperitoneal administration, the 14C in the kidneys and liver of the mother was about 50% dinoseb and 50% metabolites. However, the 14C in the kidneys and liver of the embryo was 85% dinoseb after oral administration and 57% after intraperitoneal administration.
Toxicity
Dinoseb is highly toxic when ingesting,inhaling
Breathing (or ventilation) is the process of moving air into and from the lungs to facilitate gas exchange with the internal environment, mostly to flush out carbon dioxide and bring in oxygen.
All aerobic creatures need oxygen for cellula ...
or at skin contact. Symptoms include fatigue, sweating, headaches, nausea, stomach aches and fever. It is also an irritant for the eyes. Skin contact causes burns and it turns yellow. For pregnant women this substance is especially dangerous as it can cause growth defects in unborn children (it is teratogenic
Teratology is the study of abnormalities of physiological development in organisms during their life span. It is a sub-discipline in medical genetics which focuses on the classification of congenital abnormalities in dysmorphology. The related ...
).
Dinoseb interferes with the oxidative phosphorylation
Oxidative phosphorylation (UK , US ) or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing chemical energy in order to produce adenosine t ...
by acting as an uncoupler An uncoupler or uncoupling agent is a molecule that disrupts oxidative phosphorylation in prokaryotes and mitochondria or photophosphorylation in chloroplasts and cyanobacteria by dissociating the reactions of ATP synthesis from the electron transp ...
, which is the production of ATP in the mitochondria
A mitochondrion (; ) is an organelle found in the cells of most Eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used ...
. This is done by making the inner membrane of the mitochondria more permeable to protons. The protons can return to the mitochondrial matrix more easily, which results in a lower difference in proton concentration on either side of the inner mitochondrial membrane. In other words: The proton gradient is lower, so the membrane potential will be lower. As the membrane potential is the driving force for the production of ATP, the cell is unable to produce energy.
Exposure to dinoseb also induces ER-mediated calcium release, resulting in increased intracellular calcium levels. This is followed by activation of caspase
Caspases (cysteine-aspartic proteases, cysteine aspartases or cysteine-dependent aspartate-directed proteases) are a family of protease enzymes playing essential roles in programmed cell death. They are named caspases due to their specific cyste ...
, which is a protease involved in cell apoptosis. The surviving cells have an increase of alpha-synuclein levels which leads to dopaminergic neurodegeneration.
Dinoseb can cross biological membranes like the blood-brain barrier and the placental barrier. This explains why dinoseb is particularly dangerous for pregnant women. If the compound can pass the placental barrier, the unborn child will be exposed to dinoseb via the blood of the mother.
Oral LD50 values of dinoseb range from 14 to 114 mg/kg in rats, mice, rabbits, and guinea pigs. For humans, this is 5–50 mg/kg.
Effects on animals
Dinoseb is not only a toxic compound for human but also for animals such as rats, fish and birds. Rats: Dinoseb causes acute toxicity in rats after a single dose after circa fourteen days. About 50% of the rats die when they are given 25–28 mg/kg dinoseb orally. Much more is needed when the rats are exposed to dinoseb via the skin. In this case 50% dies when the rats are exposed to 80 mg/kg. When the dinoseb is injected a dose of 20 mg/kg will cause death of 50% of the rats. But also for rats turned out dinoseb was capable to go through the placenta and therefore causes embryotoxic andteratogenic
Teratology is the study of abnormalities of physiological development in organisms during their life span. It is a sub-discipline in medical genetics which focuses on the classification of congenital abnormalities in dysmorphology. The related ...
effects.
Fish: Dinoseb is also highly toxic for fish, because fish are able to take up dinoseb very quickly. For small fish like goldfish only 0,4 ppm is needed to kill all fish in the water. When a fish lives in an acidic water environment dinoseb is more toxic than when a fish lives in a neutral or alkaline water environment. This is because dinoseb is slightly acidic.
Birds: It was found that dinoseb was also highly toxic to birds. When birds are given a single dose between 7–9 mg/kg around 50% of the birds dies. The most birds are exposed to dinoseb via the small streams of water.
Research has also shown that dinoseb is carcinogen
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive sub ...
ic for female mice, but not for male mice.
First aid measures
Nowadays dinoseb is banned in a lot of places in the world due to high incidences of birth defects. Because of this ban not a lot of people are exposed to dinoseb anymore. But when someone is exposed, a few things can be done as first aid. The victim can be exposed via four ways: inhalation, skin, eyes, ingestion. These are the first aid measures for the four ways of exposure: Inhalation: The victim should get some fresh air. When needed the victim can be administered oxygen and assisted ventilation.Bronchospasm
Bronchospasm or a bronchial spasm is a sudden constriction of the muscles in the walls of the bronchioles. It is caused by the release (degranulation) of substances from mast cells or basophils under the influence of anaphylatoxins. It causes di ...
can be treated with beta2 agonist and corticosteroid
Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are inv ...
aerosols.
Skin: The contaminated clothing and jewellery should be removed from the victim. The victim's skin, hair and nails should be washed thoroughly with soap several times.
Eyes: The victim's eyes should be immediately rinsed with running water. This is needed for at least 20 minutes. The contact lenses should be removed if possible.
Ingestion: The victim's mouth should be rinsed at first. The victim should be given charcoal
Charcoal is a lightweight black carbon residue produced by strongly heating wood (or other animal and plant materials) in minimal oxygen to remove all water and volatile constituents. In the traditional version of this pyrolysis process, ...
as a slurry (240 ml water/30 g charcoal). This is only possible when the victim is conscious.
In all of the four cases the victim should see a doctor.
Chemistry
Synthesis
The first step in the synthesis of dinoseb is the synthesis of 2-(1-methylpropyl)phenol from1-butene
1-Butene (or 1-Butylene) is the organic compound with the formula CH3CH2CH=CH2. It is a colorless gas that is easily condensed to give a colorless liquid. It is classified as a linear alpha-olefin. It is one of the isomers of butene (butylene). ...
and phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it r ...
. First, 1-butene
1-Butene (or 1-Butylene) is the organic compound with the formula CH3CH2CH=CH2. It is a colorless gas that is easily condensed to give a colorless liquid. It is classified as a linear alpha-olefin. It is one of the isomers of butene (butylene). ...
is protonated so that a secondary carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encount ...
is formed. This can only happen under acidic conditions. The formed carbocation can undergo electrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic n ...
with phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it r ...
. The product of this reaction is 2-(1-methylpropyl)phenol.
: The second step in the synthesis of dinoseb is the
The second step in the synthesis of dinoseb is the nitration
In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols an ...
of 2-(1-methylpropyl)phenol. First, the nitronium ion
The nitronium ion, , is a cation. It is an onium ion because its nitrogen atom has +1 charge, similar to ammonium ion . It is created by the removal of an electron from the paramagnetic nitrogen dioxide molecule , or the protonation of nitric ...
is formed from nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
and sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
.
: 2-(1-methylpropyl)phenol takes up the
2-(1-methylpropyl)phenol takes up the nitronium ion
The nitronium ion, , is a cation. It is an onium ion because its nitrogen atom has +1 charge, similar to ammonium ion . It is created by the removal of an electron from the paramagnetic nitrogen dioxide molecule , or the protonation of nitric ...
to form the arenium ion
An arenium ion in organic chemistry is a cyclohexadienyl cation that appears as a reactive intermediate in electrophilic aromatic substitution.
For historic reasons this complex is also called a Wheland intermediate, after American chemist George ...
, which has three resonance structures. Water can cleave off the additional proton to form a neutral compound.
: The product of this reaction can undergo a second
The product of this reaction can undergo a second nitration
In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols an ...
to form dinoseb.
:
Stereoisomerism
Dinoseb is aracemic
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
mixture of two enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical anti ...
s.
See also
* DinoterbReferences
External links
US EPA datasheet
{{Herbicides Herbicides Dinitrophenols Uncoupling agents