|
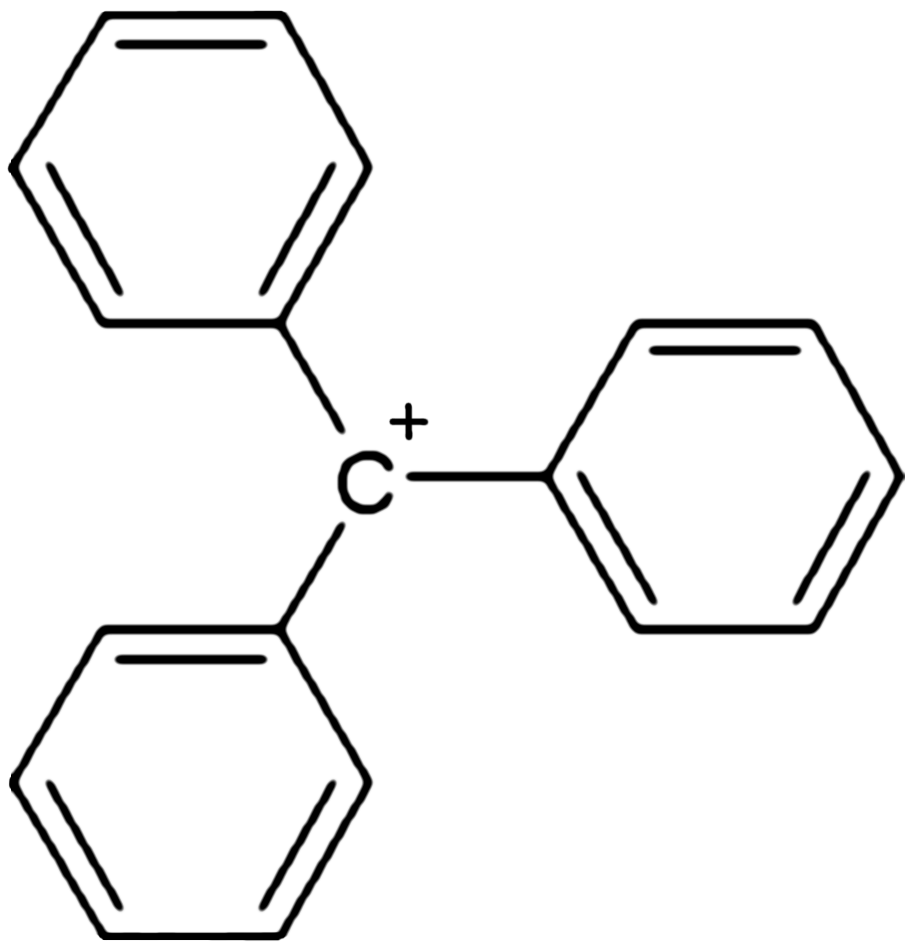
Triphenylmethyl Cation
In chemistry, triphenylcarbenium, triphenylmethyl cation, tritylium , or trityl cation is an ion with formula or , consisting of a carbon atom with a electric charge, positive charge connected to three phenyl groups. It is a charged version of the triphenylmethyl radical •. The name is often abbreviated to triphenylmethyl or trityl in salts, although these names also denote the chemical group in compounds like triphenylmethyl chloride that do not contain the cation. Triphenylcarbenium is a relatively stable carbenium ion, because the positive charge is partially distributed among 10 of the carbon atoms (the 3 carbon atoms in the ''ortho'' and ''para'' positions of each of the three phenyl groups, plus the central carbon atom). Derivatives The cation exists in important chemical reagents and catalysts such as triphenylmethyl hexafluorophosphate . Related salts are known with diverse anions including (), hexachloroantimonate (), and perchlorate (). This and other similar cat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylcarbenium
In chemistry, triphenylcarbenium, triphenylmethyl cation, tritylium , or trityl cation is an ion with formula or , consisting of a carbon atom with a positive charge connected to three phenyl groups. It is a charged version of the triphenylmethyl radical •. The name is often abbreviated to triphenylmethyl or trityl in salts, although these names also denote the chemical group in compounds like triphenylmethyl chloride that do not contain the cation. Triphenylcarbenium is a relatively stable carbenium ion, because the positive charge is partially distributed among 10 of the carbon atoms (the 3 carbon atoms in the ''ortho'' and ''para'' positions of each of the three phenyl groups, plus the central carbon atom). Derivatives The cation exists in important chemical reagents and catalysts such as triphenylmethyl hexafluorophosphate . Related salts are known with diverse anions including (), hexachloroantimonate (), and perchlorate (). This and other similar cations can be ob ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as a placeholder for the aryl group in chemical structure diagrams, analogous to “R” used for any organic substituent. “Ar” is not to be confused with the elemental symbol for argon. A simple aryl group is phenyl (), a group derived from benzene. Examples of other aryl groups consist of: * The tolyl group () which is derived from toluene (methylbenzene) * The xylyl group (), which is derived from xylene (dimethylbenzene) * The naphthyl group (), which is derived from naphthalene Arylation is the process in which an aryl group is attached to a substituent. It is typically achieved by cross-coupling reactions. Nomenclature The most basic aryl group is phenyl, which is made up of a benzene ring with one hydrogen atom substituted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylmethane
Triphenylmethane, or triphenyl methane, is the hydrocarbon with the formula (C6H5)3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane is the basic skeleton of many synthetic dyes called triarylmethane dyes, many of them are pH indicators, and some display fluorescence. A trityl group in organic chemistry is a triphenylmethyl group Ph3C, e.g. triphenylmethyl chloride (trityl chloride) and the triphenylmethyl radical (trityl radical). Preparation Triphenylmethane was first synthesized in 1872 by the German chemist August Kekulé and his Belgian student Antoine Paul Nicolas Franchimont (1844–1919) by heating diphenylmercury (Hg(C6H5)2, ''Quecksilberdiphenyl'') with benzal chloride (C6H5CHCl2, ''Benzylenchlorid''). Triphenylmethane can be synthesized by Friedel–Crafts reaction from benzene and chloroform with aluminium chloride catalyst: :3 C6H6 + CHCl3 → Ph3CH + 3 HCl Alternatively, benzene may react with carbon tetrachloride us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pararosaniline
Pararosaniline, Basic Red 9, or C.I. 42500 is an organic compound with the formula H2NC6H4)3Cl. It is a magenta solid with a variety of uses as a dye. It is one of the four components of basic fuchsine. (The others are rosaniline, new fuchsine and magenta II.) It is structurally related to other triarylmethane dyes called methyl violets including crystal violet, which feature methyl groups on nitrogen. It is prepared by the condensation of aniline and para-aminobenzaldehyde. Alternatively, it arises from the oxidation of 4,4'-bis(aminophenyl)methane in the presence of aniline. Uses *It is used to dye polyacrylonitrile fibers. *Pararosaniline is used as a colorimetric test for aldehydes, in the Schiff test. It is the only basic fuchsine component suitable for making the aldehyde-fuchsine stain for pancreatic islet beta cells. *It has use as an Antischistosomal. Related compounds * 4,4'-Thiodianiline * 4,4'-Methylenedianiline * 4,4'-Oxydianiline * Dapsone Dapsone, also kn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
New Fuchsine
New fuchsine is an organic compound with the formula H2N(CH3)C6H3)3Cl. It is a green-colored solid that is used as a dye of the triarylmethane class. It is one of the four components of basic fuchsine, and one of the two that are available as single dyes. The other is pararosaniline. It is prepared by condensation of ortho-toluidine with formaldehyde. This process initially gives the benzhydrol 4,4'-bis(dimethylamino)benzhydrol, which is further condensed to give the leuco (colorless) tertiary alcohol [(H2N(CH3)C6H3)3COH, which is oxidized in acid to give the dye. Use as dye and stain New fuchsine is used to dye polyacrylonitrile, paper, and leather. In biology, it can be used for staining (biology), staining acid-fast organisms, e.g. by Ziehl–Neelsen stain, and for making Schiff's reagent. As a primary amine, the dye can be diazotized in the laboratory, and the resulting diazonium salt used as a trapping agent in enzyme histochemistry.Lojda Z, Gossrau R, Schiebler TH ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Violet
Crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triarylmethane dye used as a histological stain and in Gram's method of classifying bacteria. Crystal violet has antibacterial, antifungal, and anthelmintic ( vermicide) properties and was formerly important as a topical antiseptic. The medical use of the dye has been largely superseded by more modern drugs, although it is still listed by the World Health Organization. The name ''gentian violet'' was originally used for a mixture of methyl pararosaniline dyes (methyl violet), but is now often considered a synonym for ''crystal violet''. The name refers to its colour, being like that of the petals of certain gentian flowers; it is not made from gentians or violets. Production A number of possible routes can be used to prepare crystal violet. The original procedure developed by the German chemists Kern and Caro involved the reaction of dimethylaniline with phosgene to gi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wiley-VCH
Wiley-VCH is a German publisher owned by John Wiley & Sons. It was founded in 1921 as Verlag Chemie (meaning "Chemistry Press": VCH stands for ''Verlag Chemie'') by two German learned societies. Later, it was merged into the German Chemical Society (GDCh). In 1991, VCH acquired Akademie Verlag :''There also were unrelated publishing houses in Stuttgart and in (East-)Berlin, and there is the (JAVG).'' Akademie Verlag (AV) is a German scientific and academic publishing company, founded in 1946 in the Soviet-occupied eastern part .... It has been owned by John Wiley & Sons since 1996. The humanities section of Akademie Verlag and the Akademie brand were sold in 1997 to R. Oldenbourg Verlag, while VCH retained the natural sciences catalog. References External links * Wiley (publisher) Publishing companies of Germany Publishing companies established in 1921 Weinheim German companies established in 1921 {{publish-company-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ullmann's Encyclopedia Of Industrial Chemistry
''Ullmann's Encyclopedia of Industrial Chemistry'' is a major reference work related to industrial chemistry by Chemist Fritz Ullmann, first published in 1914, and exclusively in German as "Enzyklopädie der Technischen Chemie" until 1984. History Ullmann's Encyclopedia of Industrial Chemistry is a major reference work related to industrial chemistry by chemist Fritz Ullmann. Its 1st edition was published in German by Fritz Ullmann in 1914. The 4th edition, published 1972 to 1984, already contained 25 volumes. The 5th edition, published 1985 to 1996, was the first version available in English. In 1997, the first online version was published. 2014 marked its centenary. As of 2016, Ullmann's Encyclopedia was in its 7th edition, in 40 volumes including one index volume and more than 1,050 articles (200 more than the 6th edition), approx. 30,000 pages, 22,000 images, 8,000 tables, 19,000 references and 85,000 indices. Editions * 1914–1922: 1st edition in 12 volumes, which can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triarylmethane Dye
Triarylmethane dyes are synthetic organic compounds containing triphenylmethane backbones. As dyes, these compounds are intensely colored. They are produced industrially as dyes. Families Triarylmethane dyes can be grouped into families according to the nature of the substituents on the aryl groups. In some cases, the anions associated with the cationic dyes (say crystal violet) vary even though the name of the dye does not. Often it is shown as chloride. Methyl violet dyes Methyl violet dyes have dimethylamino groups at the ''p''-positions of two aryl groups. Image:Methyl Violet 2B.png, Methyl violet 2B Image:Methyl Violet 6B.png, Methyl violet 6B Image:Methyl Violet 10B.png, Methyl violet 10B Fuchsine dyes Fuchsine dyes have primary or secondary amines (NH2 or NHMe) functional groups at the ''p''-positions of each aryl group. File:Pararosaniline.png, Pararosaniline File:Rosaniline hydrochloride.svg, Fuchsine (hydrochloride salt) Neofuchsin.svg, New fuchsine (As chlorid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula . It is a colorless, odorless and viscous liquid that is miscible with water. Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air. Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid, but to the contrary dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus the reverse procedure of adding water to the acid should not be performed since the heat released may boi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, volatile, colourless, flammable liquid with a distinctive alcoholic odour similar to that of ethanol (potable alcohol). A polar solvent, methanol acquired the name wood alcohol because it was once produced chiefly by the destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl benzoate, anisole, peroxyacids, as well as a host of more specialised chemicals. Occurrence Small amounts of methanol are present in normal, healthy hu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |