Triphenylmethyl Cation on:
[Wikipedia]
[Google]
[Amazon]
 In
In
Image:Methyl Violet 10B.png, Crystal violet.
Image:NewFuchsineStructure.png,
N. C. Deno, J. J. Jaruzelski, and Alan Schriesheim (1955) "Carbonium ions. I. An acidity function (''C''0) derived from arylcarbonium ion equilibria." ''Journal of the American Chemical Society'', voume 77, issue 11, pages 3044–3051.
Michael E. Jung, Roman Lagoutte, and Ullrich Jahn (2011): "Triphenylcarbenium Tetrafluoroborate". In ''Encyclopedia of Reagents for Organic Synthesis''.
E. Molins, M. Mas, W. Maniukiewicz, M. Ballester and J. Castañer (1996): "Perchlorotriphenylcarbenium Hexachloroantimonate(V)". ''Acta Crystallographica Section C (Structural Chemistry)'', volume C52, pages 2412-2414. {{doi, 10.1107/S0108270196007287
U. S. National Institutes of Health (2019)
PubChem ID 2723954 - Triphenylcarbenium hexafluorophosphate
. Entry in NCBI's PubChem database, accessed on 2019-07-25.
Carbocations

 In
In chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
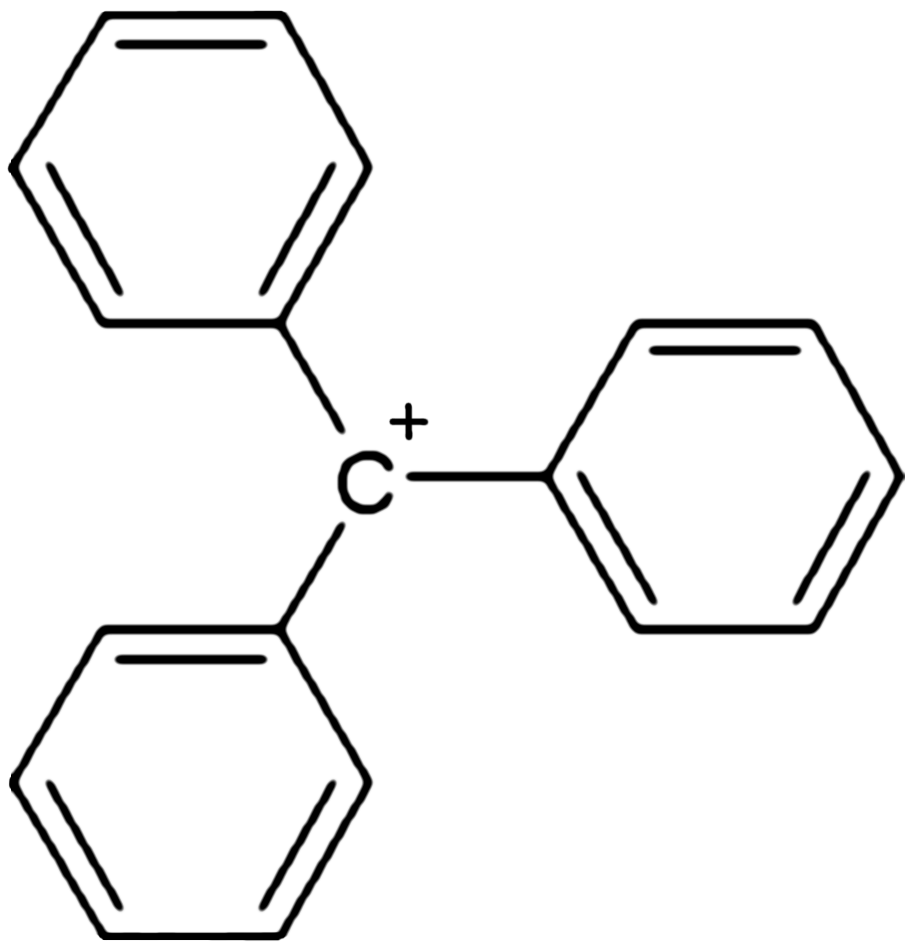
, triphenylcarbenium, triphenylmethyl cation, tritylium , or trityl cation is an ion with formula or , consisting of a carbon atom with a positive charge connected to three phenyl groups. It is a charged version of the triphenylmethyl radical •. The name is often abbreviated to triphenylmethyl or trityl in salts, although these names also denote the chemical group in compounds like triphenylmethyl chloride that do not contain the cation.
Triphenylcarbenium is a relatively stable carbenium
A carbenium ion is a positive ion with the structure RR′R″C+, that is, a chemical species with a trivalent carbon that bears a +1 formal charge.
In older literature the name carbonium ion was used for this class, but now it refers exclusivel ...
ion, because the positive charge is partially distributed among 10 of the carbon atoms (the 3 carbon atoms in the ''ortho'' and ''para'' positions of each of the three phenyl groups, plus the central carbon atom).
Derivatives
The cation exists in important chemicalreagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
s and catalysts such as triphenylmethyl hexafluorophosphate . Related salts are known with diverse anions including (), hexachloroantimonate
Antimony pentachloride is a chemical compound with the formula SbCl5. It is a colourless oil, but typical samples are yellowish due to dissolved chlorine. Owing to its tendency to hydrolyse to hydrochloric acid, SbCl5 is a highly corrosive substa ...
(), and perchlorate (). This and other similar cations can be obtained as intensely colored solutions by dissolving aryl-substituted methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
s in concentrated sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
. Derivatives of this cation include, for example, perchlorotriphenylcarbenium .
Triarylmethane dyes
Triarylmethane dyes are derivatives are stabilized version of the trityl cation. They are water-soluble and are often obtained as the chloride salts. These dyes have strong electron donor groups, often amines, at the ''p''-positions of two or three of the aryl groups.Thomas Gessner and Udo Mayer "Triarylmethane and Diarylmethane Dyes" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim.New fuchsine
New fuchsine is an organic compound with the formula H2N(CH3)C6H3)3Cl. It is a green-colored solid that is used as a dye of the triarylmethane class. It is one of the four components of basic fuchsine, and one of the two that are available as ...
dye.
File:Pararosaniline.png, Pararosaniline
See also
* Triphenylmethane * TriphenylmethanolReferences
PubChem ID 2723954 - Triphenylcarbenium hexafluorophosphate
. Entry in NCBI's PubChem database, accessed on 2019-07-25.