|
TRAMP Complex
TRAMP complex (Trf4/Air2/Mtr4p Polyadenylation complex) is a multiprotein, heterotrimeric complex having distributive polyadenylation activity and identifies wide varieties of RNAs produced by polymerases. It was originally discovered in ''Saccharomyces'' ''cerevisiae'' by LaCava et al., Vanacova et al. and Wyers et al. in 2005. It interacts with the exosome complex in the nucleus of eukaryotic cells and is involved in the 3' end processing and degradation of ribosomal RNA and snoRNAs. The TRAMP complex trims the poly(A) tails of RNAs destined foRrp6and the core exosome down to 4-5 adenosines assisting in transcript recognition and exosome complex activation. The substrate specificity of exosomes is improved in the presence of TRAMP complex as it acts as a crucial cofactor and helps in maintaining various activities. In this way, TRAMP plays a critical role in ridding the cell of noncoding transcripts generated through pervasive RNA polymerase II transcription, as well as function ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyadenylation
Polyadenylation is the addition of a poly(A) tail to an RNA transcript, typically a messenger RNA (mRNA). The poly(A) tail consists of multiple adenosine monophosphates; in other words, it is a stretch of RNA that has only adenine bases. In eukaryotes, polyadenylation is part of the process that produces mature mRNA for translation. In many bacteria, the poly(A) tail promotes degradation of the mRNA. It, therefore, forms part of the larger process of gene expression. The process of polyadenylation begins as the transcription of a gene terminates. The 3′-most segment of the newly made pre-mRNA is first cleaved off by a set of proteins; these proteins then synthesize the poly(A) tail at the RNA's 3′ end. In some genes these proteins add a poly(A) tail at one of several possible sites. Therefore, polyadenylation can produce more than one transcript from a single gene (alternative polyadenylation), similar to alternative splicing. The poly(A) tail is important for the nuclea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RNA Recognition Motif
RNA recognition motif, RNP-1 is a putative RNA-binding domain of about 90 amino acids that are known to bind single-stranded RNAs. It was found in many eukaryotic proteins. The largest group of single strand RNA-binding protein is the eukaryotic RNA recognition motif (RRM) family that contains an eight amino acid RNP-1 consensus sequence. RRM proteins have a variety of RNA binding preferences and functions, and include heterogeneous nuclear ribonucleoproteins (hnRNPs), proteins implicated in regulation of alternative splicing (SR, U2AF2, Sxl), protein components of small nuclear ribonucleoproteins (U1 and U2 snRNPs), and proteins that regulate RNA stability and translation ( PABP, La, Hu). The RRM in heterodimeric splicing factor U2 snRNP auxiliary factor appears to have two RRM-like domains with specialised features for protein recognition. The motif also appears in a few single stranded DNA binding proteins. The typical RRM consists of four anti-parallel beta-strands and t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
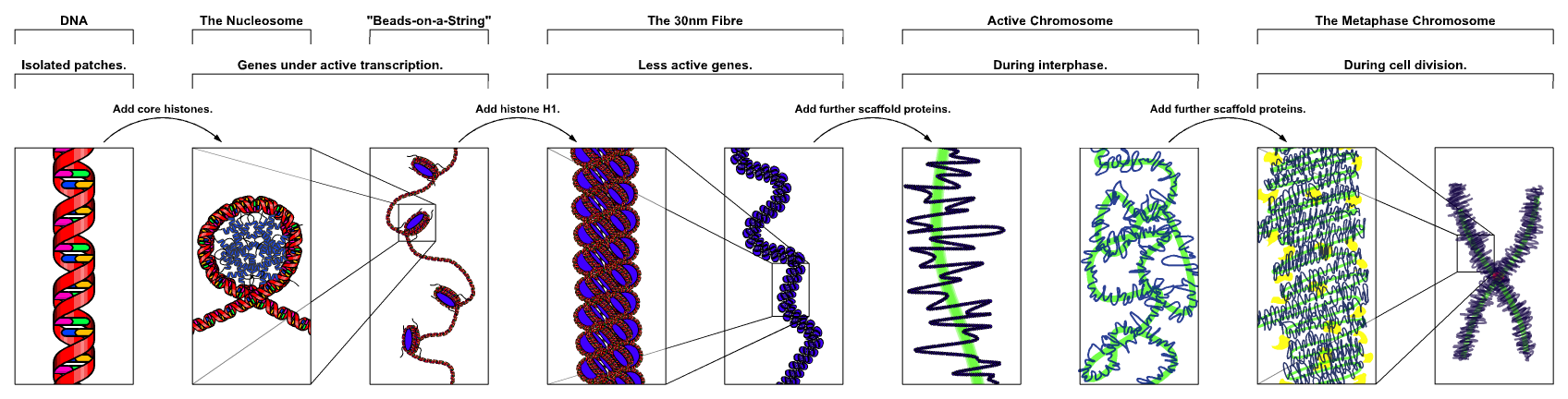
Chromatin
Chromatin is a complex of DNA and protein found in eukaryotic cells. The primary function is to package long DNA molecules into more compact, denser structures. This prevents the strands from becoming tangled and also plays important roles in reinforcing the DNA during cell division, preventing DNA damage, and regulating gene expression and DNA replication. During mitosis and meiosis, chromatin facilitates proper segregation of the chromosomes in anaphase; the characteristic shapes of chromosomes visible during this stage are the result of DNA being coiled into highly condensed chromatin. The primary protein components of chromatin are histones. An octamer of two sets of four histone cores (Histone H2A, Histone H2B, Histone H3, and Histone H4) bind to DNA and function as "anchors" around which the strands are wound.Maeshima, K., Ide, S., & Babokhov, M. (2019). Dynamic chromatin organization without the 30-nm fiber. ''Current opinion in cell biology, 58,'' 95–104. https://doi.o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyltransferase
Methyltransferases are a large group of enzymes that all methylate their substrates but can be split into several subclasses based on their structural features. The most common class of methyltransferases is class I, all of which contain a Rossmann fold for binding ''S''-Adenosyl methionine (SAM). Class II methyltransferases contain a SET domain, which are exemplified by SET domain histone methyltransferases, and class III methyltransferases, which are membrane associated. Methyltransferases can also be grouped as different types utilizing different substrates in methyl transfer reactions. These types include protein methyltransferases, DNA/RNA methyltransferases, natural product methyltransferases, and non-SAM dependent methyltransferases. SAM is the classical methyl donor for methyltransferases, however, examples of other methyl donors are seen in nature. The general mechanism for methyl transfer is a SN2-like nucleophilic attack where the methionine sulfur serves as the leavi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Small Nuclear RNA
Small nuclear RNA (snRNA) is a class of small RNA molecules that are found within the splicing speckles and Cajal bodies of the cell nucleus in eukaryotic cells. The length of an average snRNA is approximately 150 nucleotides. They are transcribed by either RNA polymerase II or RNA polymerase III. Their primary function is in the processing of pre-messenger RNA (hnRNA) in the nucleus. They have also been shown to aid in the regulation of transcription factors (7SK RNA) or RNA polymerase II (B2 RNA), and maintaining the telomeres. snRNA are always associated with a set of specific proteins, and the complexes are referred to as small nuclear ribonucleoproteins (snRNP, often pronounced "snurps"). Each snRNP particle is composed of a snRNA component and several snRNP-specific proteins (including Sm proteins, a family of nuclear proteins). The most common human snRNA components of these complexes are known, respectively, as: U1 spliceosomal RNA, U2 spliceosomal RNA, U4 spliceosomal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transfer RNA
Transfer RNA (abbreviated tRNA and formerly referred to as sRNA, for soluble RNA) is an adaptor molecule composed of RNA, typically 76 to 90 nucleotides in length (in eukaryotes), that serves as the physical link between the mRNA and the amino acid sequence of proteins. tRNAs genes from Bacteria are typically shorter (mean = 77.6 bp) than tRNAs from Archaea (mean = 83.1 bp) and eukaryotes (mean = 84.7 bp). The mature tRNA follows an opposite pattern with tRNAs from Bacteria being usually longer (median = 77.6 nt) than tRNAs from Archaea (median = 76.8 nt), with eukaryotes exhibiting the shortest mature tRNAs (median = 74.5 nt). Transfer RNA (tRNA) does this by carrying an amino acid to the protein synthesizing machinery of a cell called the ribosome. Complementation of a 3-nucleotide codon in a messenger RNA (mRNA) by a 3-nucleotide anticodon of the tRNA results in protein synthesis based on the mRNA code. As such, tRNAs are a necessary component of translation, the biological sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Small Nucleolar RNA
In molecular biology, Small nucleolar RNAs (snoRNAs) are a class of small RNA molecules that primarily guide chemical modifications of other RNAs, mainly ribosomal RNAs, transfer RNAs and small nuclear RNAs. There are two main classes of snoRNA, the C/D box snoRNAs, which are associated with methylation, and the H/ACA box snoRNAs, which are associated with pseudouridylation. SnoRNAs are commonly referred to as guide RNAs but should not be confused with the guide RNAs that direct RNA editing in trypanosomes. snoRNA guided modifications After transcription, nascent rRNA molecules (termed pre-rRNA) undergo a series of processing steps to generate the mature rRNA molecule. Prior to cleavage by exo- and endonucleases, the pre-rRNA undergoes a complex pattern of nucleoside modifications. These include methylations and pseudouridylations, guided by snoRNAs. *Methylation is the attachment or substitution of a methyl group onto various substrates. The rRNA of humans contain approximately ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ski Complex
The Ski complex is a multi-protein complex involved in the 3' end degradation of messenger RNAs in yeast. Structure The complex consists of three main proteins, the RNA helicase ''Ski2'' and the proteins ''Ski3'' and ''Ski8''. This tetramer contains a 370 kDa core complex, containing N-terminal arms and C-terminal arms from ''Ski3''. The helicase core of ''Ski2'' is positioned by both the C-terminal of ''Ski3'' and two subunits of ''Ski8''. Mechanism Helicase activities are initiated by the N-terminal arm and the ''Ski2'' insertion domain. In yeast, the complex guides RNA molecules to the exosome complex for degradation via a fourth protein, called ''Ski7'', which contains a GTPase-like protein. ''Ski7'' involves the 3’ to 5’ degradation of RNA through two different pathways, 3’ poly(A) tail shortening and the binding of the ''Ski2'', ''Ski3'', and ''Ski8'' tetramer and the exosome. Degradation of the 3' mRNA overhang occurs by association with the 80s ribosome. The 3' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transforming Growth Factor Beta Superfamily
The transforming growth factor beta (TGF-β) superfamily is a large group of structurally related cell regulatory proteins that was named after its first member, TGF-β1, originally described in 1983. They interact with TGF-beta receptors. Many proteins have since been described as members of the TGF-β superfamily in a variety of species, including invertebrates as well as vertebrates and categorized into 23 distinct gene types that fall into four major subfamilies: * The TGF-β subfamily * The bone morphogenetic proteins and the growth differentiation factors * The activin and inhibin subfamilies * The left-right determination factors * A group encompassing various divergent members Transforming growth factor-beta (TGF-beta) is a multifunctional peptide that controls proliferation, differentiation and other functions in many cell types. TGF-beta-1 is a peptide of 112 amino acid residues derived by proteolytic cleavage from the C-terminal of a precursor protein. These pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helix Bundle
A helix bundle is a small protein fold composed of several alpha helices that are usually nearly parallel or antiparallel to each other. Three-helix bundles Three-helix bundles are among the smallest and fastest known cooperatively folding structural domains. The three-helix bundle in the villin headpiece domain is only 36 amino acids long and is a common subject of study in molecular dynamics simulations because its microsecond-scale folding time is within the timescales accessible to simulation. The 40-residue HIV accessory protein has a very similar fold and has also been the subject of extensive study. There is no general sequence motif associated with three-helix bundles, so they cannot necessarily be predicted from sequence alone. Three-helix bundles often occur in actin-binding proteins and in DNA-binding proteins. Four-helix bundles Four-helix bundles typically consist of four helices packed in a coiled-coil arrangement with a sterically close-packed hydrophobic core in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helix-turn-helix
Helix-turn-helix is a DNA-binding protein (DBP). The helix-turn-helix (HTH) is a major structural motif capable of binding DNA. Each monomer incorporates two α helices, joined by a short strand of amino acids, that bind to the major groove of DNA. The HTH motif occurs in many proteins that regulate gene expression. It should not be confused with the helix–loop–helix motif. Discovery The discovery of the helix-turn-helix motif was based on similarities between several genes encoding transcription regulatory proteins from bacteriophage lambda and ''Escherichia coli'': Cro, CAP, and λ repressor, which were found to share a common 20–25 amino acid sequence that facilitates DNA recognition. Function The helix-turn-helix motif is a DNA-binding motif. The recognition and binding to DNA by helix-turn-helix proteins is done by the two α helices, one occupying the N-terminal end of the motif, the other at the C-terminus. In most cases, such as in the Cro repressor, the second ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |