|
Two-dimensional Infrared Spectroscopy
Two-dimensional infrared spectroscopy (2D IR) is a nonlinear infrared spectroscopy technique that has the ability to correlate vibrational modes in condensed-phase systems. This technique provides information beyond linear infrared spectra, by spreading the vibrational information along multiple axes, yielding a frequency correlation spectrum. A frequency correlation spectrum can offer structural information such as vibrational mode coupling, anharmonicities, along with chemical dynamics such as energy transfer rates and molecular dynamics with femtosecond time resolution. 2DIR experiments have only become possible with the development of ultrafast lasers and the ability to generate femtosecond infrared pulses. Systems studied Among the many systems studied with infrared spectroscopy are water, metal carbonyls, short polypeptides, proteins, perovskite solar cells, and DNA oligomers. Experimental approaches There are two main approaches to two-dimensional spectroscopy, the Fouri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Picosecond
A picosecond (abbreviated as ps) is a unit of time in the International System of Units (SI) equal to 10−12 or (one trillionth) of a second. That is one trillionth, or one millionth of one millionth of a second, or 0.000 000 000 001 seconds. A picosecond is to one second as one second is to approximately 31,689 years. Multiple technical approaches achieve imaging within single-digit picoseconds: for example, the streak camera or intensified CCD (ICCD) cameras are able to picture the motion of light. One picosecond is equal to 1000 femtoseconds, or 1/1000 nanoseconds. Because the next SI unit is 1000 times larger, measurements of 10−11 and 10−10 second are typically expressed as tens or hundreds of picoseconds. Some notable measurements in this range include: * 1.0 picoseconds (1.0 ps) – cycle time for electromagnetic frequency 1 terahertz (THz) (1 x 1012 hertz), an inverse unit. This corresponds to a wavelength of 0.3 mm, as can be calculated by m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shaul Mukamel
Shaul Mukamel (born 1948) is a chemist and physicist, currently serving as a Distinguished Professor at the University of California, Irvine. He is known for his works in Non linear Optics and Spectroscopy. Early life and education Shaul Mukamel was born in Baghdad, Iraq on December 11, 1948. Mukamel received his B.Sc. degree in 1969, with the distinction cum laude and his M.Sc. and Ph.D., both summa cum laude, in 1971 and 1976 respectively from Tel Aviv University. His Masters supervisor was Uzi Kaldor. He finished his PhD working under Joshua Jortner. Following graduation, Mukamel served as postdoc at MIT and the University of California, Berkeley. Career Mukamel has worked at Rice University and the Weizmann Institute before joining University of Rochester, where he worked from 1982 to 2003. He has been at University of California, Irvine since then. Mukamel is known for his work in the field of nonlinear optics, especially the time domain extensions which culminated in the w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition State
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked with the double dagger ‡ symbol. As an example, the transition state shown below occurs during the SN2 reaction of bromoethane with a hydroxide anion: The activated complex of a reaction can refer to either the transition state or to other states along the reaction coordinate between reactants and products, especially those close to the transition state.Peter Atkins and Julio de Paula, ''Physical Chemistry'' (8th ed., W.H. Freeman 2006), p.809 According to the transition state theory, once the reactants have passed through the transition state configuration, they always continue to form products. History of concept The concept of a transition state has been important in many theories of the rates at which chemical reactions occ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted , where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are the second-row elements nitrogen (N), oxygen (O), and fluorine (F). Hydrogen bonds can be intermolecular (occurring between separate molecules) or intramolecular (occurring among parts of the same molecule). The energy of a hydrogen bond depends on the geometry, the environment, and the nature of the specific donor and acceptor atoms and can vary between 1 and 40 kcal/mol. This makes them somewhat stronger than a van der Waals interaction, and weaker than fully covalent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Motional Narrowing
In physics and chemistry, motional narrowing is a phenomenon where a certain resonant frequency has a smaller linewidth than might be expected, due to motion in an inhomogeneous system.''Solid state: nuclear methods'' by J. N. Mundy, section 6.2.1.1, page 441. The discovery of motional narrowing has been attributed to during his thesis work in the 1940s Example: NMR spectroscopy A common example is NMR. In this process, the |
Excited State
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to an increase in energy level above a chosen starting point, usually the ground state, but sometimes an already excited state. The temperature of a group of particles is indicative of the level of excitation (with the notable exception of systems that exhibit negative temperature). The lifetime of a system in an excited state is usually short: spontaneous or induced emission of a quantum of energy (such as a photon or a phonon) usually occurs shortly after the system is promoted to the excited state, returning the system to a state with lower energy (a less excited state or the ground state). This return to a lower energy level is often loosely described as decay and is the inverse of excitation. Long-lived excited states are often called ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schematic Of Typical Two-dimensional Infrared Spectrum
A schematic, or schematic diagram, is a designed representation of the elements of a system using abstract, graphic symbols rather than realistic pictures. A schematic usually omits all details that are not relevant to the key information the schematic is intended to convey, and may include oversimplified elements in order to make this essential meaning easier to grasp, as well as additional organization of the information. For example, a subway map intended for passengers may represent a subway station with a dot. The dot is not intended to resemble the actual station at all but aims to give the viewer information without unnecessary visual clutter. A schematic diagram of a chemical process uses symbols in place of detailed representations of the vessels, piping, valves, pumps, and other equipment that compose the system, thus emphasizing the functions of the individual elements and the interconnections among them and suppresses their physical details. In an electronic circuit d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectrometer
A spectrometer () is a scientific instrument used to separate and measure spectral components of a physical phenomenon. Spectrometer is a broad term often used to describe instruments that measure a continuous variable of a phenomenon where the spectral components are somehow mixed. In visible light a spectrometer can separate white light and measure individual narrow bands of color, called a spectrum. A mass spectrometer measures the spectrum of the masses of the atoms or molecules present in a gas. The first spectrometers were used to split light into an array of separate colors. Spectrometers were developed in early studies of physics, astronomy, and chemistry. The capability of spectroscopy to determine chemical composition drove its advancement and continues to be one of its primary uses. Spectrometers are used in astronomy to analyze the chemical composition of stars and planets, and spectrometers gather data on the origin of the universe. Examples of spectrometers are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
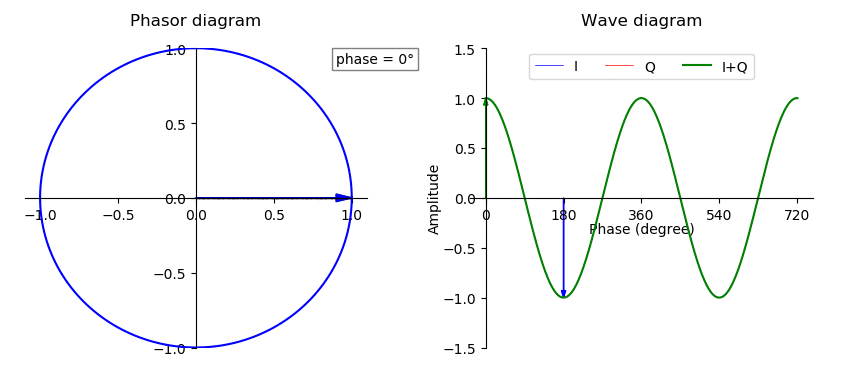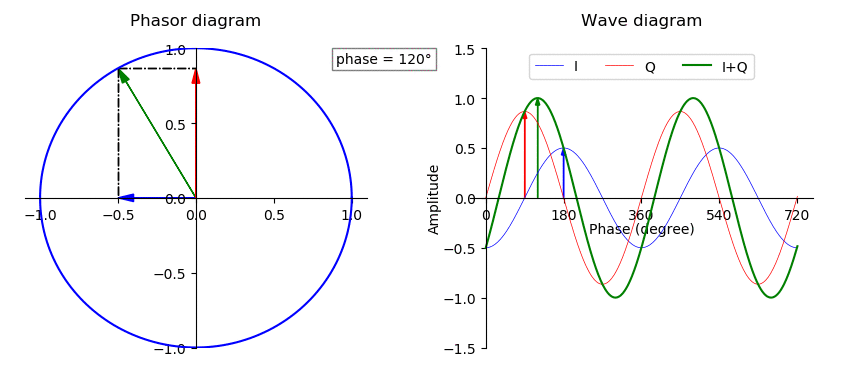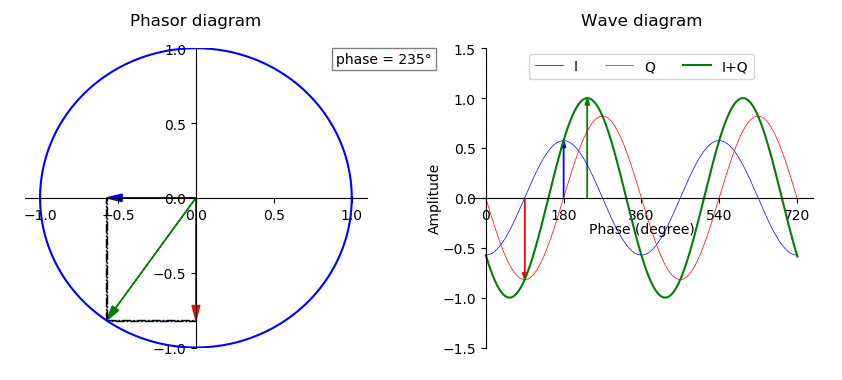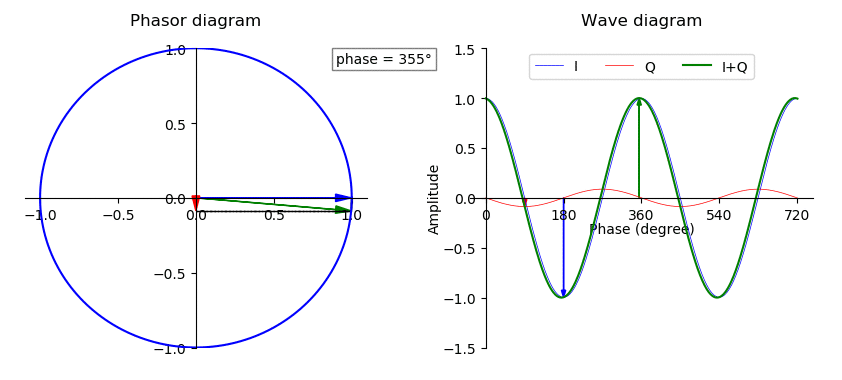
Phase (waves)
In physics and mathematics, the phase of a periodic function F of some real variable t (such as time) is an angle-like quantity representing the fraction of the cycle covered up to t. It is denoted \phi(t) and expressed in such a scale that it varies by one full turn as the variable t goes through each period (and F(t) goes through each complete cycle). It may be measured in any angular unit such as degrees or radians, thus increasing by 360° or 2\pi as the variable t completes a full period. This convention is especially appropriate for a sinusoidal function, since its value at any argument t then can be expressed as \phi(t), the sine of the phase, multiplied by some factor (the amplitude of the sinusoid). (The cosine may be used instead of sine, depending on where one considers each period to start.) Usually, whole turns are ignored when expressing the phase; so that \phi(t) is also a periodic function, with the same period as F, that repeatedly scans the same range of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frequency
Frequency is the number of occurrences of a repeating event per unit of time. It is also occasionally referred to as ''temporal frequency'' for clarity, and is distinct from ''angular frequency''. Frequency is measured in hertz (Hz) which is equal to one event per second. The period is the interval of time between events, so the period is the reciprocal of the frequency. For example, if a heart beats at a frequency of 120 times a minute (2 hertz), the period, —the interval at which the beats repeat—is half a second (60 seconds divided by 120 beats). Frequency is an important parameter used in science and engineering to specify the rate of oscillatory and vibratory phenomena, such as mechanical vibrations, audio signals (sound), radio waves, and light. Definitions and units For cyclical phenomena such as oscillations, waves, or for examples of simple harmonic motion, the term ''frequency'' is defined as the number of cycles or vibrations per unit of time. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterodyne
A heterodyne is a signal frequency that is created by combining or mixing two other frequencies using a signal processing technique called ''heterodyning'', which was invented by Canadian inventor-engineer Reginald Fessenden. Heterodyning is used to shift signals from one frequency range into another, and is also involved in the processes of modulation and demodulation. The two input frequencies are combined in a nonlinear signal-processing device such as a vacuum tube, transistor, or diode, usually called a '' mixer''. In the most common application, two signals at frequencies and are mixed, creating two new signals, one at the sum of the two frequencies , and the other at the difference between the two frequencies . The new signal frequencies are called ''heterodynes''. Typically, only one of the heterodynes is required and the other signal is filtered out of the output of the mixer. Heterodyne frequencies are related to the phenomenon of "beats" in acoustics. A major a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |