|
Trifluoromethyl Hypofluorite
Trifluoromethyl hypofluorite is an organofluorine compound with the formula . It exists as a colorless gas at room temperature and is highly toxic. It is a rare example of a hypofluorite (compound with an O−F bond). It is prepared by the reaction of fluorine gas with carbon monoxide: :2 F2 + CO → CF3OF The gas hydrolyzes only slowly at neutral pH. Use in organic chemistry The compound is a source of electrophilic fluorine. It has been used for the preparation of α-fluoroketones from silyl enol ethers. Behaving like a pseudohalogen, it adds to ethylene to give the ether In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be c ...: :CF3OF + CH2CH2 → CF3OCH2CH2F References {{Reflist Trifluoromethoxy compounds Fluorinating agents Hypofluorites ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organofluorine Compound
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents. The carbon–fluorine bond Fluorine has several distinctive differences from all other substituents encountered in organic molecules. As a result, the physical and chemical properties of organofluorines can be distinctive in comparison to other organohalogens. # The carbon–fluorine bond is one of the strongest in organic chemistry (an average bond energy around 480 kJ/molKirsch, Peer ''Modern fluoroorga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypofluorous Acid
Hypofluorous acid, chemical formula H O F, is the only known oxyacid of fluorine and the only known oxoacid in which the main atom gains electrons from oxygen to create a negative oxidation state. The oxidation state of the oxygen in hypofluorites is 0. It is also the only hypohalous acid that can be isolated as a solid. HOF is an intermediate in the oxidation of water by fluorine, which produces hydrogen fluoride, oxygen difluoride, hydrogen peroxide, ozone and oxygen. HOF is explosive at room temperature, forming HF and O2: :2 HOF → 2 HF + O2 It was isolated in the pure form by passing F2 gas over ice at −40 °C, collecting the HOF gas, and condensing it: :F2 + H2O → HOF + HF The compound has been characterized in the solid phase by X-ray crystallography as a bent molecule with an angle of 101°. The O–F and O–H bond lengths are 144.2 and 96.4 picometres, respectively. The solid framework consists of chains with O–H···O linkages. The structure has also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for modern pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest molecule of the oxocarbon family. In coordination complexes the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds, when insufficient oxygen or heat is present to produce carbon dioxide. There are also numerous environmental and biological sources that generate and emit a significant amount of carbon monoxide. It is important in the production of many compounds, including drugs, fragrances, and fuels. Upon emission into the atmosphere, carbon monoxide affects several processes that contribute to climate change. Carbon monoxide has important biological roles across phylogenetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons. Electrophiles mainly interact with nucleophiles through addition and substitution reactions. Frequently seen electrophiles in organic syntheses include cations such as H+ and NO+, polarized neutral molecules such as HCl, alkyl halides, acyl halides, and carbonyl compounds, polarizable neutral molecules such as Cl2 and Br2, oxidizing agents such as organic peracids, chemical species that do not satisfy the octet rule such as carbenes and radicals, and some Lewis acids such as BH3 and DIBAL. Organic chemistry Addition of halogens These occur between alkenes and electrophiles, often halogens as in halogen addition reactions. Common reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haloketone
In organic chemistry, an α-haloketone is a functional group consisting of a ketone group or more generally a carbonyl group with an α-halogen substituent. α-haloketones are alkylating agents. Prominent α-haloketones include phenacyl bromide and chloroacetone. Structure The general structure is RR′C(X)C(=O)R where R is an alkyl or aryl residue and X any one of the halogens. The preferred conformation of a haloketone is that of a cisoid with the halogen and carbonyl sharing the same plane as the steric hindrance with the carbonyl alkyl group is generally larger. Haloketone synthesis Haloketones and halo carbonyl compounds in general are synthesized by reaction of carbonyl compounds with sources of X+ (X = halogen), which is provided using halogens: :RC(O)CH3 + X2 → RC(O)CH2X + HX Specialized sources of electrophilic halogenating agents include ''N''-Bromosuccinimide and 1,3-dibromo-5,5-dimethylhydantoin (DBDMH). In the Nierenstein reaction an acyl chloride re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
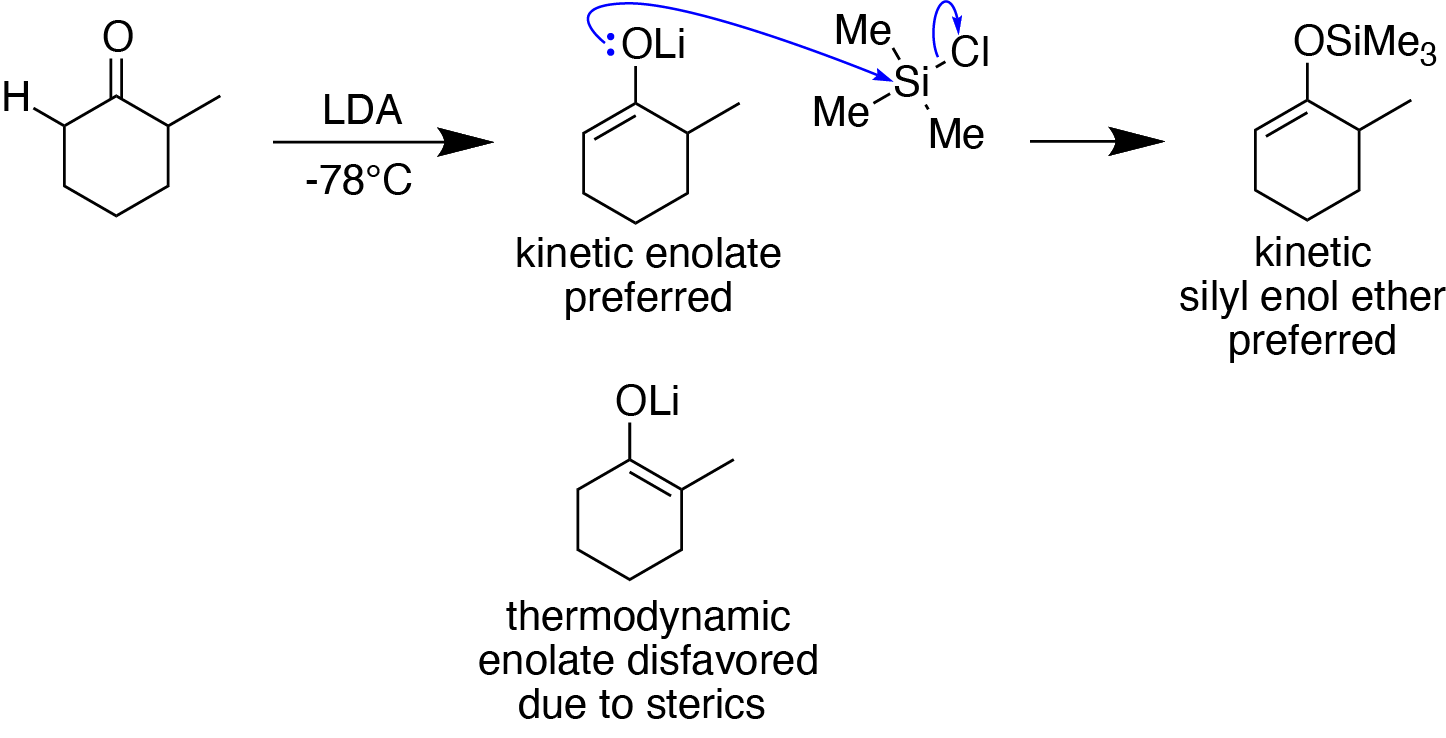
Silyl Enol Ethers
Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen end to an organosilicon group. They are important intermediates in organic synthesis. Synthesis Silyl enol ethers are generally prepared by reacting an enolizable Carbonyl group, carbonyl compound with a silyl electrophile and a Base (chemistry), base, or just reacting an enolate with a silyl electrophile.Clayden, J., Greeves, N., & Warren, S. (2012). Silyl enol ethers. In ''Organic chemistry'' (Second ed., pp. 466-467). Oxford University Press. Since silyl electrophiles are HSAB theory, hard and silicon-oxygen bonds are very strong, the oxygen (of the carbonyl compound or enolate) acts as the nucleophile to form a Si-O single bond. The most commonly used silyl electrophile is trimethylsilyl chloride. To increase the rate of reaction, trimethylsilyl triflate may also be used in the place of trimethylsilyl chloride as a more el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudohalogen
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorganic molecules of the general forms ''Ps''–''Ps'' or ''Ps''–X (where ''Ps'' is a pseudohalogen group), such as cyanogen; pseudohalide anions, such as cyanide ion; inorganic acids, such as hydrogen cyanide; as ligands in coordination complexes, such as ferouscyanide; and as functional groups in organic molecules, such as the nitrile group. Well-known pseudohalogen functional groups include cyanide, cyanate, thiocyanate, and azide. Common pseudohalogens and their nomenclature Many pseudohalogens are known by specialized common names according to where they occur in a compound. Well-known ones include (the true halogen chlorine is listed for comparison): Au− is considered to be a pseudohalogen ion due to its disproportionation reactio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds). Ethylene is widely used in the chemical industry, and its worldwide production (over 150 million tonnes in 2016) exceeds that of any other organic compound. Much of this production goes toward polyethylene, a widely used plastic containing polymer chains of ethylene units in various chain lengths. Ethylene is also an important natural plant hormone and is used in agriculture to force the ripening of fruits. The hydrate of ethylene is ethanol. Structure and properties This hydrocarbon has four hydrogen atoms bound to a pair of carbon atoms that are connected by a double bond. All six atoms that comprise ethylene are coplanar. The H-C-H angle is 117.4°, close to the 120° for ideal sp² hybridized carbon. The molecule is also relatively weak: rota ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl or aryl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin. Structure and bonding Ethers feature bent C–O–C linkages. In dimethyl ether, the bond angle is 111° and C–O distances are 141 pm. The barrier to rotation about the C–O bonds is low. The bonding of oxygen in ethers, alcohols, and water is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoromethoxy Compounds
The trifluoromethoxy group is the chemical group –O–. It can be seen as a methoxy group –O– whose hydrogen atoms are replaced by fluorine atoms; or as a trifluoromethyl group attached to the rest of the molecule by a bridging oxygen atom.''Synthetic Approaches to Trifluoromethoxy-Substituted Compounds'' A. Tlili, F. Toulgoat, T. Billard, Angew. Chem. Int. Ed. 2016, 55, 11726–11735; Compounds having this functional group are of some relevance as pharmaceuticals. One example is riluzole Riluzole is a medication used to treat amyotrophic lateral sclerosis and other motor neuron diseases. Riluzole delays the onset of ventilator-dependence or tracheostomy in some people and may increase survival by two to three months. Riluzole is .... References {{organohalide-stub Haloalkyl groups ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |